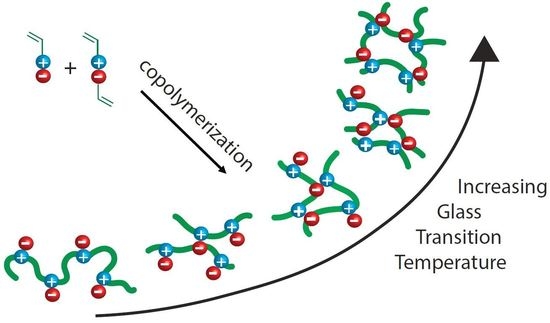
Systematic Modification of the Glass Transition Temperature of Ion-Pair Comonomer Based Polyelectrolytes and Ionomers by Copolymerization with a Chemically Similar Cationic Monomer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Monomer and Polymer Synthesis
2.2. Thermo-Mechanical Behavior
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Zhang, Y.; Batys, P.; O’Neal, J.T.; Li, F.; Sammalkorpi, M.; Lutkenhaus, J.L. Molecular Origin of the Glass Transition in Polyelectrolyte Assemblies. ACS Cent. Sci. 2018, 4, 638–644. [Google Scholar] [CrossRef]
- Potaufeux, J.-E.; Odent, J.; Notta-Cuvier, D.; Lauro, F.; Raquez, J.-M. A comprehensive review of the structures and properties of ionic polymeric materials. Polym. Chem. 2020, 11, 5914–5936. [Google Scholar] [CrossRef]
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef] [Green Version]
- Shamoun, R.F.; Hariri, H.H.; Ghostine, R.A.; Schlenoff, J.B. Thermal Transformations in Extruded Saloplastic Polyelectrolyte Complexes. Macromolecules 2012, 45, 9759–9767. [Google Scholar] [CrossRef]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer Research and Applications. Macromol. React. Eng. 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Hemp, S.T.; Zhang, M.; Allen, M.H.; Cheng, S.; Moore, R.B.; Long, T.E. Comparing Ammonium and Phosphonium Polymerized Ionic Liquids: Thermal Analysis, Conductivity, and Morphology. Macromol. Chem. Phys. 2013, 214, 2099–2107. [Google Scholar] [CrossRef]
- Scott, P.J.; Spiering, G.A.; Wang, Y.; Seibers, Z.D.; Moore, R.B.; Kumar, R.; Lokitz, B.S.; Long, T.E. Phosphonium-Based Polyzwitterions: Influence of Ionic Structure and Association on Mechanical Properties. Macromolecules 2020, 53, 11009–11018. [Google Scholar] [CrossRef]
- Cui, J.; Nie, F.-M.; Yang, J.-X.; Pan, L.; Ma, Z.; Li, Y.-S. Novel Imidazolium-Based Poly(Ionic Liquid)s with Different Counterions for Self-Healing. J. Mater. Chem. A 2017, 5, 25220–25229. [Google Scholar] [CrossRef]
- Hunley, M.T.; England, J.P.; Long, T.E. Influence of Counteranion on the Thermal and Solution Behavior of Poly(2-(dimethylamino)ethyl methacrylate)-Based Polyelectrolytes. Macromolecules 2010, 43, 9998–10005. [Google Scholar] [CrossRef]
- Weiss, R.A.; Agarwal, P.K.; Lundberg, R.D. Control of ionic interactions in sulfonated polystyrene ionomers by the use of alkyl-substituted ammonium counterions. J. Appl. Polym. Sci. 1984, 29, 2719–2734. [Google Scholar] [CrossRef]
- Manoj Lalwani, S.; Eneh, C.I.; Lutkenhaus, J.L. Emerging trends in the dynamics of polyelectrolyte complexes. Phys. Chem. Chem. Phys. 2020, 22, 24157–24177. [Google Scholar] [CrossRef]
- Fu, J.; Fares, H.M.; Schlenoff, J.B. Ion-Pairing Strength in Polyelectrolyte Complexes. Macromolecules 2017, 50, 1066–1074. [Google Scholar] [CrossRef]
- Yang, M.; Digby, Z.A.; Schlenoff, J.B. Precision Doping of Polyelectrolyte Complexes: Insight on the Role of Ions. Macromolecules 2020, 53, 5465–5474. [Google Scholar] [CrossRef]
- Saikaew, R.; Meesorn, W.; Zoppe, J.O.; Weder, C.; Dubas, S.T. Influence of the Salt Concentration on the Properties of Salt-Free Polyelectrolyte Complex Membranes. Macromol. Mater. Eng. 2019, 304, 1900245. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Antila, H.S.; Lutkenhaus, J.L.; Sammalkorpi, M. Role of Salt and Water in the Plasticization of PDAC/PSS Polyelectrolyte Assemblies. J. Phys. Chem. B 2017, 121, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, F.; Valenzuela, L.D.; Sammalkorpi, M.; Lutkenhaus, J.L. Effect of Water on the Thermal Transition Observed in Poly(allylamine hydrochloride)-Poly(acrylic acid) Complexes. Macromolecules 2016, 49, 7563–7570. [Google Scholar] [CrossRef]
- Schaaf, P.; Schlenoff, J.B. Saloplastics: Processing Compact Polyelectrolyte Complexes. Adv. Mater. 2015, 27, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Peterson, A.M. Humidity Tempering of Polyelectrolyte Complexes. Macromolecules 2018, 51, 10003–10010. [Google Scholar] [CrossRef]
- Batys, P.; Zhang, Y.; Lutkenhaus, J.L.; Sammalkorpi, M. Hydration and Temperature Response of Water Mobility in Poly(diallyldimethylammonium)-Poly(sodium 4-styrenesulfonate) Complexes. Macromolecules 2018, 51, 8268–8277. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Abbett, R.L.; Fares, H.M.; Schlenoff, J.B. Water and the Glass Transition Temperature in a Polyelectrolyte Complex. ACS Macro Lett. 2017, 6, 1114–1118. [Google Scholar] [CrossRef]
- Yildirim, E.; Zhang, Y.; Lutkenhaus, J.L.; Sammalkorpi, M. Thermal Transitions in Polyelectrolyte Assemblies Occur via a Dehydration Mechanism. ACS Macro Lett. 2015, 4, 1017–1021. [Google Scholar] [CrossRef]
- Salamone, J.C.; Watterson, A.C.; Quach, L.; Raheja, M.K. Polymerization of ion-pair comonomers. Polym. Prepr. Am. Chem. Soc. Div. Polym. Chem. 1985, 26, 196–197. [Google Scholar]
- Neculescu, C.; Clough, S.B.; Elayaperumal, P.; Salamone, J.C.; Watterson, A.C. Thermal analysis of ampholytic polymers. J. Polym. Sci. Part C Polym. Lett. 1987, 25, 201–203. [Google Scholar] [CrossRef]
- Salamone, J.C.; Raheja, M.K.; Anwaruddin, Q.; Watterson, A.C. Polymerization of vinylpyridinium salts. XIII. Preparation of 4-vinyl-N-methylpyridinium p-styrenesulfonate charge transfer ion-pair comonomer. J. Polym. Sci. Polym. Lett. Ed. 1985, 23, 655–659. [Google Scholar] [CrossRef]
- Salamone, J.C.; Quach, L.; Watterson, A.C.; Krauser, S.; Mahmud, M.U. Polymerization of ion-pair comonomers of related structures. J. Macromol. Sci. Chem. 1985, A22, 653–664. [Google Scholar] [CrossRef]
- Clough, S.B.; Cortelek, D.; Nagabhushanam, T.; Salamone, J.C.; Watterson, A.C. Small angle scattering from ampholytic styrene ionomers. Polym. Eng. Sci. 1984, 24, 385–390. [Google Scholar] [CrossRef]
- Salamone, J.C.; Mahmud, N.A.; Mahmud, M.U.; Nagabhushanam, T.; Watterson, A.C. Acrylic ampholytic ionomers. Polymer 1982, 23, 843–848. [Google Scholar] [CrossRef]
- Salamone, J.C.; Watterson, A.C.; Hsu, T.D.; Tsai, C.C.; Mahmud, M.U. Polymerization of vinylpyridinium salts. IX. Preparation of monomeric salt pairs. J. Polym. Sci. Polym. Lett. Ed. 1977, 15, 487–491. [Google Scholar] [CrossRef]
- Wickramasinhage, R.N.; Goswami, S.; McAdam, C.J.; Hanton, L.R.; Moratti, S.C. Tough polymeric hydrogels using ion-pair comonomers. Soft Matter 2020, 16, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, L.; Yang, C.; Yang, Y.; Song, C.; Wu, Q.; Huang, G.; Wu, J. Super tough and strong self-healing elastomers based on polyampholytes. J. Mater. Chem. A 2018, 6, 19066–19074. [Google Scholar] [CrossRef]
- Sun, T.L.; Kurokawa, T.; Kuroda, S.; Ihsan, A.B.; Akasaki, T.; Sato, K.; Haque, M.A.; Nakajima, T.; Gong, J.P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Cavicchi, K.A. Tuning the Viscoelastic Properties of Poly(n-butyl acrylate) Ionomer Networks through the Use of Ion-Pair Comonomers. Macromolecules 2017, 50, 9473–9481. [Google Scholar] [CrossRef]
- Jin, K.; Torkelson, J.M. Enhanced Tg-Confinement Effect in Cross-Linked Polystyrene Compared to Its Linear Precursor: Roles of Fragility and Chain Architecture. Macromolecules 2016, 49, 5092–5103. [Google Scholar] [CrossRef]
- Goswami, M.; Kumar, S.K.; Bhattacharya, A.; Douglas, J.F. Computer Simulations of Ionomer Self-Assembly and Dynamics. Macromolecules 2007, 40, 4113–4118. [Google Scholar] [CrossRef]
- Eisenberg, A.; Hird, B.; Moore, R.B. A new multiplet-cluster model for the morphology of random ionomers. Macromolecules 1990, 23, 4098–4107. [Google Scholar] [CrossRef]
- Lu, K.; Maranas, J.K.; Milner, S.T. Depletion attraction of sheet-like ion aggregates in low-dielectric ionomer melts. J. Chem. Phys. 2017, 146, 064901–064908. [Google Scholar] [CrossRef]
- Chremos, A.; Douglas, J.F. Polyelectrolyte association and solvation. J. Chem. Phys. 2018, 149, 163305. [Google Scholar] [CrossRef]
- Peng, B.; Yang, Y.; Gu, K.; Amis, E.J.; Cavicchi, K.A. Digital Light Processing 3D Printing of Triple Shape Memory Polymer for Sequential Shape Shifting. ACS Mater. Lett. 2019, 1, 410–417. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Jones, A.S.; Wright, T.; Smook, M.A.; Harwood, H.J. Enhancement of the high-temperature utility of a polystyrene-b-poly(ethylene-co-butylene)-b-polystyrene block copolymer by friedel–crafts naphthoylation. J. Appl. Polym. Sci. 2003, 88, 1289–1295. [Google Scholar] [CrossRef]
- Wright, T.; Jones, A.S.; James Harwood, H. Enhancement of the high-temperature properties of an SEBS thermoplastic elastomer by chemical modification. J. Appl. Polym. Sci. 2002, 86, 1203–1210. [Google Scholar] [CrossRef]
- Hemp, S.T.; Zhang, M.; Tamami, M.; Long, T.E. Phosphonium ionenes from well-defined step-growth polymerization: Thermal and melt rheological properties. Polym. Chem. 2013, 4, 3582–3590. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Poly-mer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Gordon, M.; Taylor, J.S. Ideal Copolymers and the Second-Order Transitions of Synthetic Rubbers. I. Noncrystalline Copolymers. Rubber Chem. Technol. 1953, 26, 323–335. [Google Scholar] [CrossRef]
- Gordon, M.; Taylor, J.S. Ideal copolymers and the second-order transitions of synthetic rubbers. i. non-crystalline copolymers. J. Appl. Chem. 1952, 2, 493–500. [Google Scholar] [CrossRef]
- Couchman, P.; Karasz, F. A classical thermodynamic discussion of the effect of composition on glass-transition temperatures. Macromolecules 1978, 11, 117–119. [Google Scholar] [CrossRef]
- Zhang, L.H.; Cool, L.R.; Wesdemiotis, C.; Weiss, R.A.; Cavicchi, K.A. Syntheses of quaternary ammonium-containing, trithiocarbonate RAFT agents and hemi-telechelic cationomers. Polym. Chem. 2014, 5, 1180–1190. [Google Scholar] [CrossRef]







| Sample | VBTOP-SS (mmoL) | VBTOP-TS (mmoL) | RAFT (mmoL) | AIBN (mg) | BzCl (g) | State after Reaction | Yield |
|---|---|---|---|---|---|---|---|
| PIPC-CM-1-0 | 1.0 | 0 | 0.025 | 0.82 | 2.98 | Gel | 98% |
| PIPC-CM-0.75-0.25 | 0.75 | 0.25 | 0.025 | 0.82 | 2.98 | Gel | 94% |
| PIPC-CM-0.5-0.5 | 0.5 | 0.5 | 0.025 | 0.82 | 2.98 | Solution | 95% |
| PIPC-CM-0.25-0.75 | 0.25 | 0.75 | 0.025 | 0.82 | 2.98 | Solution | 98% |
| PIPC-CM-0-1 | 0 | 1.0 | 0.025 | 0.82 | 2.98 | Solution | 94% |
| Sample | VBTOP-SS (mmoL) | VBTOP-TS (mmoL) | n-BA (mmoL) | RAFT (mmoL) | AIBN (mg) | BzCl (g) | State after Reaction | Yield |
|---|---|---|---|---|---|---|---|---|
| PBA-IPC-CM-10-1-0 | 1.0 | 0 | 10 | 0.05 | 1.64 | 2.98 | Gel | 98% |
| PBA-IPC-CM-10-0.75-0.25 | 0.75 | 0.25 | 10 | 0.05 | 1.64 | 2.98 | Gel | 96% |
| PBA-IPC-CM-10-0.5-0.5 | 0.5 | 0.5 | 10 | 0.05 | 1.64 | 2.98 | Solution | 97% |
| PBA-IPC-CM-10-0.25-0.75 | 0.25 | 0.75 | 10 | 0.05 | 1.64 | 2.98 | Solution | 96% |
| PBA-IPC-CM-10-0-1 | 0 | 1.0 | 10 | 0.05 | 1.64 | 2.98 | Solution | 95% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, G.; Schoch, T.D.; Cavicchi, K.A. Systematic Modification of the Glass Transition Temperature of Ion-Pair Comonomer Based Polyelectrolytes and Ionomers by Copolymerization with a Chemically Similar Cationic Monomer. Gels 2021, 7, 45. https://doi.org/10.3390/gels7020045
Deng G, Schoch TD, Cavicchi KA. Systematic Modification of the Glass Transition Temperature of Ion-Pair Comonomer Based Polyelectrolytes and Ionomers by Copolymerization with a Chemically Similar Cationic Monomer. Gels. 2021; 7(2):45. https://doi.org/10.3390/gels7020045
Chicago/Turabian StyleDeng, Guodong, Timothy D. Schoch, and Kevin A. Cavicchi. 2021. "Systematic Modification of the Glass Transition Temperature of Ion-Pair Comonomer Based Polyelectrolytes and Ionomers by Copolymerization with a Chemically Similar Cationic Monomer" Gels 7, no. 2: 45. https://doi.org/10.3390/gels7020045
APA StyleDeng, G., Schoch, T. D., & Cavicchi, K. A. (2021). Systematic Modification of the Glass Transition Temperature of Ion-Pair Comonomer Based Polyelectrolytes and Ionomers by Copolymerization with a Chemically Similar Cationic Monomer. Gels, 7(2), 45. https://doi.org/10.3390/gels7020045