Tuning the Mechanical and Thermal Properties of Hydroxypropyl Methylcellulose Cryogels with the Aid of Surfactants
Abstract
:1. Introduction
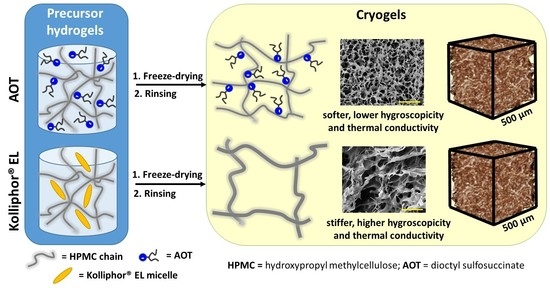
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of HPMC Cryogels
4.3. Characterization of Precursor Hydrogels
4.4. Characterization of the Cryogels
4.5. Density Functional Theory (DFT) Calculations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mansour, F.; Waheed, S.; Paull, B.; Maya, F. Porogens and Porogen Selection in the Preparation of Porous Polymer Monoliths. J. Sep. Sci. 2019, 43, 56–69. [Google Scholar] [CrossRef]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50† Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef] [Green Version]
- Buchtová, N.; Budtova, T. Cellulose Aero-, Cryo- and Xerogels: Towards Understanding of Morphology Control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of Aerogels, Cryogels and Xerogels of Cellulose with Hierarchical Porous Structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Blachechen, L.; Fardim, P.; Petri, D. Multifunctional Cellulose Beads and Their Interaction with GramPositive Bacteria. Biomacromolecules 2014, 15, 3440–3448. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Budtova, T. Polysaccharide-Based Aerogels for Thermal Insulation and Superinsulation: An Overview. Carbohydr. Polym. 2021, 266, 118130. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Yoda, S. Chitosan Aerogels: Transparent, Flexible Thermal Insulators. Chem. Mater. 2015, 27, 7569–7572. [Google Scholar] [CrossRef]
- Nešić, A.; Gordić, M.; Davidovic, S.; Radovanović, Ž.; Nedeljković, J.; Smirnova, I.; Gurikov, P. Pectin-Based Nanocomposite Aerogels for Potential Insulated Food Packaging Application. Carbohydr. Polym. 2018, 195, 128–135. [Google Scholar] [CrossRef]
- Rudaz, C.; Courson, R.; Bonnet, L.; Calas-Etienne, S.; Sallée, H.; Budtova, T. Aeropectin: Fully Biomass-Based Mechanically Strong and Thermal Superinsulating Aerogel. Biomacromolecules 2014, 15, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- Lavoine, N.; Bergström, L. Nanocellulose-Based Foams and Aerogels: Processing, Properties, and Applications. J. Mater. Chem. A 2017, 5, 16105–16117. [Google Scholar] [CrossRef] [Green Version]
- Ni, X.; Ke, F.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F. The Control of Ice Crystal Growth and Effect on Porous Structure of Konjac Glucomannan-Based Aerogels. Int. J. Biol. Macromol. 2016, 92, 1130–1135. [Google Scholar] [CrossRef]
- Kaushiva, B.; McCartney, S.; Rossmy, G.; Wilkes, G. Surfactant Level Influences on Structure and Properties of Flexible Slabstock Polyurethane Foams. Polymer 2000, 41, 285–310. [Google Scholar] [CrossRef]
- Li, Y.; Tolley, H.D.; Lee, M.L. Preparation of Polymer Monoliths That Exhibit Size Exclusion Properties for Proteins and Peptides. Anal. Chem. 2009, 81, 4406–4413. [Google Scholar] [CrossRef]
- Tundisi, L.L.; Mostaço, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl Methylcellulose: Physicochemical Properties and Ocular Drug Delivery Formulations. Eur. J. Pharm. Sci. 2021, 159, 105736. [Google Scholar] [CrossRef] [PubMed]
- Burdock, G.A. Safety Assessment of Hydroxypropyl Methylcellulose as a Food Ingredient. Food Chem. Toxicol. 2007, 45, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Petrov, P.; Petrova, E.; Stamenova, R.; Tsvetanov, C.B.; Riess, G. Cryogels of Cellulose Derivatives Prepared via UV irradiAtion of Moderately Frozen Systems. Polymer 2006, 47, 6481–6484. [Google Scholar] [CrossRef]
- Martins, B.; Toledo, P.; Petri, D. Hydroxypropyl Methylcellulose Based Aerogels: Synthesis, Characterization and Application as Adsorbents for Wastewater Pollutants. Carbohyd. Polym. 2017, 155, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J.K. (Ed.) Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions, 16th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 866–867. [Google Scholar]
- Nave, S.; Paul, A.; Eastoe, J.; Pitt, A.R.; Heenan, R. What Is So Special about Aerosol-OT? Part IV. Phenyl-Tipped Surfactants. Langmuir 2005, 21, 10021–10027. [Google Scholar] [CrossRef]
- Marani, P.; Bloisi, G.; Petri, D. Hydroxypropylmethyl Cellulose Films Crosslinked with Citric Acid for Control Release of Nic-otine. Cellulose 2015, 22, 173–181. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Avalle, M.; Belingardi, G.; Montanini, R. Characterization of Polymeric Structural Foams under Compressive Impact Loading by Means of Energy-Absorption Diagram. Int. J. Impact Eng. 2001, 25, 455–472. [Google Scholar] [CrossRef]
- Bajwa, G.S.; Sammon, C.; Timmins, P.; Melia, C.D. Molecular and Mechanical Properties of Hydroxypropyl Methylcellulose Solutions during the Sol-Gel Transition. Polymer 2009, 50, 4571–4576. [Google Scholar] [CrossRef]
- Odgaard, A.; Gundersen, H.J. Quantification of Connectivity in Cancellous Bone, with Special Emphasis on 3-D Reconstruc-tions. Bone 1993, 14, 173–182. [Google Scholar] [CrossRef]
- Apostolopoulou-Kalkavoura, V.; Gordeyeva, K.; Lavoine, N.; Bergström, L. Thermal Conductivity of Hygroscopic Foams Based on Cellulose Nanofibrils and a Nonionic Polyoxamer. Cellulose 2017, 25, 1117–1126. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Chen, Z.; Lyu, S.; Fu, F.; Wang, S. Highly Flexible Cross-Linked Cellulose Nanofibril Sponge-Like Aerogels with Improved Mechanical Property and Enhanced Flame Retardancy. Carbohydr. Polym. 2018, 179, 333–340. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Song, L.; Hu, Y. Nano-Fibrillated Cellulose-Hydroxyapatite based Composite Foams with Excellent Fire Resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Belda, R.; Palomar, M.; Marco, M.; Vercher-Martínez, A.; Giner, E. Open Cell Polyurethane Foam Compression Failure Characterization and Its Relationship to Morphometry. Mater. Sci. Eng. C 2021, 120, 111754. [Google Scholar] [CrossRef] [PubMed]
- Suys, E.J.; Warren, D.B.; Pham, A.C.; Nowell, C.J.; Clulow, A.J.; Benameur, H.; Porter, C.; Pouton, C.W.; Chalmers, D.K. A Nonionic Polyethylene Oxide (PEO) Surfactant Model: Experimental and Molecular Dynamics Studies of Kolliphor EL. J. Pharm. Sci. 2019, 108, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Mondal, P.; Khakhar, D.V. Hydraulic Resistance of Rigid Polyurethane Foams. I. Effect of Different Surfactants on Foam Structure and Properties. J. Appl. Polym. Sci. 2004, 93, 2821–2829. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Effect of Freeze-Drying Parameters on the Microstructure and Thermal Insulating Properties of Nanofibrillated Cellulose Aerogels. J. Sol-Gel. Sci. Technol. 2017, 84, 475–485. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, NY, USA, 1997. [Google Scholar]
- Das, D.; Dey, J.; Chandra, A.K.; Thapa, U.; Ismail, K. Aggregation Behavior of Sodium Dioctylsulfosuccinate in Aqueous Ethylene Glycol Medium. A Case of Hydrogen Bonding between Surfactant and Solvent and Its Manifestation in the Surface Tension Isotherm. Langmuir 2012, 28, 15762–15769. [Google Scholar] [CrossRef]
- Ueda, M.; Schelly, Z.A. Mean Aggregation Number and Water Vapor Pressure of AOT Reverse Micellar Systems Determined by Controlled Partial Pressure-Vapor Pressure Osmometry (CPP-VPO). Langmuir 1988, 4, 653–655. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J. Surface Modification of Textiles by Aqueous Solutions. In Surface Modification of Textiles; Wei, Q., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2009; pp. 269–295. [Google Scholar]
- Chatterjee, A.M.S.P.; Moulik, S.P.; Sanyal, S.K.; Mishra, B.K.; Puri, P.M. Thermodynamics of Micelle Formation of Ionic Surfactants: A Critical Assessment for Sodium Dodecyl Sulfate, Cetyl Pyridinium Chloride and Dioctyl Sulfosuccinate (Na Salt) by Microcalorimetric, Conductometric, and Tensiometric Measurements. J. Phys. Chem. B 2001, 105, 12823–12831. [Google Scholar] [CrossRef]
- Szymczyk, K.; Szaniawska, M.; Krawczyk, J. Temperature Effect on the Adsorption and Volumetric Properties of Aqueous Solutions of Kolliphor® ELP. Molecules 2020, 25, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Ma, A.; Li, Y. Transient Hot Strip Measures Thermal Conductivity of Organic Foam Thermal Insulation Materials. Exp. Therm. Fluid Sci. 2018, 91, 443–450. [Google Scholar] [CrossRef]
- Jiménez-Saelices, C.; Seantier, B.; Cathala, B.; Grohens, Y. Spray Freeze-Dried Nanofibrillated Cellulose Aerogels with Thermal Super-Insulating Properties. Carbohydr. Polym. 2017, 157, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bendahou, D.; Bendahou, A.; Seantier, B.; Grohens, Y.; Kaddami, H. Nano-Fibrillated Cellulose-Zeolites Based New Hybrid Composites Aerogels with Super Thermal Insulating Properties. Ind. Crops Prod. 2015, 65, 374–382. [Google Scholar] [CrossRef]
- Tseng, C.-J.; Yamaguchi, M.; Ohmori, T. Thermal Conductivity of Polyurethane Foams from Room Temperature to 20 K. Cryogenics 1997, 37, 305–312. [Google Scholar] [CrossRef]
- Standardization, E.C.F. Flexible Cellular Polymeric Materials—Determination of Stress-Strain Characteristics in Compression-Part 1: Low-Density Materials; EN ISO 3386-1; ISO: London, UK, 1997. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2016; Available online: https://gaussian.com/glossary/g09/ (accessed on 25 June 2021).












| Code | HPMC | K0.5 | AOT0.5 | K2.75 | AOT2.75 | K5 | AOT5 | K7.25 | AOT7.25 |
|---|---|---|---|---|---|---|---|---|---|
| [Kolliphor® EL] mM | - | 0.2 | - | 1.0 | - | 2.0 | - | 3.0 | - |
| [AOT] mM | - | - | 0.2 | - | 1.0 | - | 2.0 | - | 3.0 |
| Sample | ρap (kg/m3) | Gel (%) | (kPa) | (kPa) | tan δ |
|---|---|---|---|---|---|
| HPMC | 34 ± 3 | 86 ± 1 | 154 ± 15 | 7.7 ± 0.8 | 2.89 ± 0.09 |
| K0.5 | 33 ± 2 | 87 ± 2 | 320 ± 32 | 16 ± 2 | 2.91 ± 0.09 |
| K2.75 | 35 ± 3 | 67 ± 2 | 202 ± 20 | 10 ± 1 | 3.16 ± 0.09 |
| K5 | 37 ± 3 | 63 ± 2 | 234 ± 23 | 12 ± 1 | 2.96 ± 0.09 |
| K7.25 | 39 ± 5 | 61 ± 1 | 283 ± 28 | 14 ± 1 | 2.73 ± 0.08 |
| AOT0.5 | 32 ± 4 | 88 ± 3 | 205 ± 20 | 10 ± 1 | 2.89 ± 0.09 |
| AOT2.75 | 34 ± 3 | 86 ± 2 | 176 ± 18 | 8.8 ± 0.9 | 3.18 ± 0.09 |
| AOT5 | 31 ± 4 | 86 ± 1 | 39 ± 4 | 2.0 ± 0.2 | 3.03 ± 0.09 |
| AOT7.25 | 34 ± 2 | 84 ± 1 | 13 ± 1 | 0.65 ± 0.07 | 2.91 ± 0.09 |
| Sample | VOI (mm3) | (mm2) | Connectivity Density (mm−3) | (m2/kg) | Mean Pore Size (μm) |
|---|---|---|---|---|---|
| HPMC | 1.302 | 82.2 | 25,482 | 1856 | 34.0 ± 10.0 |
| K7.25 | 1.322 | 49.9 | 8570 | 1110 | 45.8 ± 9.0 |
| AOT7.25 | 1.273 | 99.9 | 29,900 | 2308 | 25.3 ± 9.4 |
| Sample | Δ%m | ||
|---|---|---|---|
| HPMC (powder) | 363 | 9.52 | 4.9 |
| Kolliphor (pure) | 415 | 1.84 | 2.5 |
| AOT (pure) | 293 | 15.53 | 3.8 |
| HPMC | 358 | 0.47 | 5.7 |
| K0.5 | 340 | 6.22 | 6.7 |
| K7.25 | 344 | 3.90 | 6.2 |
| AOT0.5 | 327 | 5.93 | 7.2 |
| AOT7.25 | 356 | 11.78 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dezotti, R.S.; Furtado, L.M.; Yee, M.; Valera, T.S.; Balaji, K.; Ando, R.A.; Petri, D.F.S. Tuning the Mechanical and Thermal Properties of Hydroxypropyl Methylcellulose Cryogels with the Aid of Surfactants. Gels 2021, 7, 118. https://doi.org/10.3390/gels7030118
Dezotti RS, Furtado LM, Yee M, Valera TS, Balaji K, Ando RA, Petri DFS. Tuning the Mechanical and Thermal Properties of Hydroxypropyl Methylcellulose Cryogels with the Aid of Surfactants. Gels. 2021; 7(3):118. https://doi.org/10.3390/gels7030118
Chicago/Turabian StyleDezotti, Rafael S., Laíse M. Furtado, Márcio Yee, Ticiane S. Valera, Krishnasamy Balaji, Rômulo A. Ando, and Denise F. S. Petri. 2021. "Tuning the Mechanical and Thermal Properties of Hydroxypropyl Methylcellulose Cryogels with the Aid of Surfactants" Gels 7, no. 3: 118. https://doi.org/10.3390/gels7030118
APA StyleDezotti, R. S., Furtado, L. M., Yee, M., Valera, T. S., Balaji, K., Ando, R. A., & Petri, D. F. S. (2021). Tuning the Mechanical and Thermal Properties of Hydroxypropyl Methylcellulose Cryogels with the Aid of Surfactants. Gels, 7(3), 118. https://doi.org/10.3390/gels7030118