Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study
Abstract
:1. Introduction
2. Result and Discussions
2.1. Preliminary Screening
2.2. Optimization
2.2.1. Effect of Formulation Variables on VS
2.2.2. Effect of Formulation Variables on Percent EE
2.2.3. Optimization of CIP-Loaded BLO Formulation
2.2.4. Characterization of CIP-BLO-Opt Formulation
2.2.5. Entrapment Efficiency
2.2.6. DSC Study
2.2.7. Solid-State Characterization by X-ray Diffractometer
2.2.8. Storage Stability Study
2.2.9. Preparation of CIP-BLO-Opt Loaded In-Situ Gel
2.2.10. Characterization of CIP-BLO-Opt-IG
Gelling Strength Studies
Clarity, pH, and Transmission Measurement
Viscosity Determination
Drug Content
2.2.11. Selection of Optimized CIP-BLO-Opt-IG
2.2.12. In-Vitro Release
2.2.13. Ex-Vivo Trans Corneal Permeation Study
2.2.14. Bio-Adhesive Study
2.2.15. Corneal Hydration Study
2.2.16. Histology Study
2.2.17. Ex-Vivo Ocular Tolerance Study by HET-CAM
2.2.18. Biological Compatibility Evaluation
2.2.19. Sterility Study
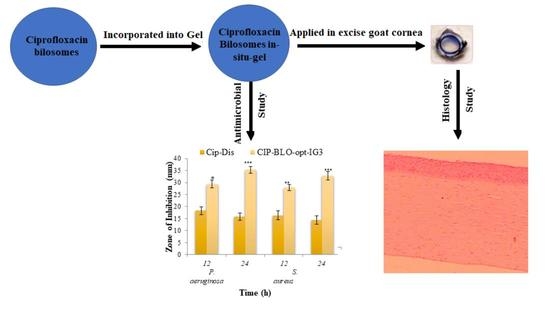
2.2.20. Antimicrobial Evaluation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of CIP-Loaded BLO
4.3. Optimization by BBD
4.4. Characterization of CIP-BLO
4.5. Entrapment Efficiency (%)
4.6. Thermal Analysis
4.7. X-ray Diffraction Study
4.8. Storage Stability Study
4.9. Preparation of CIP-BLO In-Situ Gel
4.10. Characterization of CIP-BLO In-Situ Gel
Gelling Strength
4.11. Measurement of Clarity, pH, and Transmission
4.12. Measurement of Viscosity
4.13. Determination of Drug Content
4.14. In-Vitro Dissolution Study
4.15. Ex-Vivo Corneal Permeation Study
4.16. Bio-Adhesive Study
4.17. Corneal Hydration Level (CHL) Study
4.18. Histology Study
4.19. Ex-Vivo Ocular Tolerance
4.20. Biological Compatibility Evaluation
4.21. Sterility Study
4.22. Antimicrobial Evaluation
4.23. Statistical Evaluation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Destruel, P.-L.; Zeng, N.; Seguin, J.; Douat, S.; Rosa, F.; Brignole-Baudouin, F.; Dufaÿ, S.; Dufaÿ-Wojcicki, A.; Maury, M.; Mignet, N.; et al. Novel in situ gelling ophthalmic drug delivery system based on gellan gum and hydroxyethylcellulose: Innovative rheological characterization, in vitro and in vivo evidence of a sustained precorneal retention time. Int. J. Pharm. 2020, 574, 118734. [Google Scholar] [CrossRef] [PubMed]
- Makwana, S.; Patel, V.; Parmar, S. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma Sci. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular Drug Delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.; Elsabahy, M.; El Badry, M.; Eleraky, N.E. Betaxolol-loaded niosomes integrated within pH-sensitive in situ forming gel for management of glaucoma. Int. J. Pharm. 2021, 598, 120380. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; Dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- Al-Joufi, F.A.; Salem-Bekhit, M.M.; Taha, E.I.; Ibrahim, M.A.; Muharram, M.M.; Alshehri, S.; Ghoneim, M.M.; Shakeel, F. Enhancing Ocular Bioavailability of Ciprofloxacin Using Colloidal Lipid-Based Carrier for the Management of Post-Surgical Infection. Molecules 2022, 27, 733. [Google Scholar] [CrossRef]
- Polat, H.K.; Kurt, N.; Aytekin, E.; Çaylı, Y.A.; Pehlivan, S.B.; Çalış, S. Design of Besifloxacin HCl-Loaded Nanostructured Lipid Carriers: In Vitro and Ex Vivo Evaluation. J. Ocul. Pharmacol. Ther. 2022, 38, 412–423. [Google Scholar] [CrossRef]
- Youssef, A.; Cai, C.; Dudhipala, N.; Majumdar, S. Design of Topical Ocular Ciprofloxacin Nanoemulsion for the Management of Bacterial Keratitis. Pharmaceuticals 2021, 14, 210. [Google Scholar] [CrossRef]
- Allam, A.; El-Mokhtar, M.A.; Elsabahy, M. Vancomycin-loaded niosomes integrated within pH-sensitive in-situ forming gel for treatment of ocular infections while minimizing drug irritation. J. Pharm. Pharmacol. 2019, 71, 1209–1221. [Google Scholar] [CrossRef]
- Zafar, A.; Alsaidan, O.A.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Khalid, M. Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation. Gels 2022, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; Aburahma, M.H. Investigating the potential of employing bilosomes as a novel vesicular carrier for transdermal delivery of tenoxicam. Int. J. Pharm. 2015, 485, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Conacher, M.; Alexander, J.; Brewer, J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 2001, 19, 2965–2974. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Ameeduzzafar Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alhakamy, N.A.; Alharbi, K.S.; Alshehri, S.; Afzal, M.; Alenezi, S.K.; Bukhari, S.N.A. Formulation of Chitosan Polymeric Vesicles of Ciprofloxacin for Ocular Delivery: Box-Behnken Optimization, In Vitro Characterization, HET-CAM Irritation, and Antimicrobial Assessment. AAPS PharmSciTech. 2020, 21, 167. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Khabour, O.F.; Al-Azzam, S.I. Ciprofloxacin-Induced Antibacterial Activity Is Attenuated by Phosphodiesterase Inhibitors. Curr. Ther. Res. Clin. Exp. 2015, 77, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Pignatello, R.; Leonardi, A.; Fuochi, V.; Petronio, G.P.; Greco, A.S.; Furneri, P.M. A Method for Efficient Loading of Ciprofloxacin Hydrochloride in Cationic Solid Lipid Nanoparticles: Formulation and Microbiological Evaluation. Nanomaterials 2018, 8, 304. [Google Scholar] [CrossRef] [Green Version]
- Hosny, K.M. Ciprofloxacin as Ocular Liposomal Hydrogel. AAPS PharmSciTech 2010, 11, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Takács-Novák, K.; Józan, M.; Hermecz, I.; Szász, G. Lipophilicity of antibacterial fluoroquinolones. Int. J. Pharm. 1992, 79, 89–96. [Google Scholar] [CrossRef]
- Alharbi, W.S.; Hosny, K.M. Development and optimization of ocular in situ gels loaded with ciprofloxacin cubic liquid crystalline nanoparticles. J. Drug Deliv. Sci. Technol. 2020, 57, 101710. [Google Scholar] [CrossRef]
- Gupta, S.; Vyas, S.P. Carbopol/chitosan based pH triggered in situ gelling system for ocular delivery of timolol maleate. Sci Pharm. 2010, 78, 959–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheshala, R.; Kok, Y.; Ng, J.; Thakur, R.; Dua, K. In Situ Gelling Ophthalmic Drug Delivery System: An Overview and Its Applications. Recent Patents Drug Deliv. Formul. 2015, 9, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Kouchak, M.; Mahmoodzadeh, M.; Farrahi, F. Designing of a pH-Triggered Carbopol®/HPMC In Situ Gel for Ocular Delivery of Dorzolamide HCl: In Vitro, In Vivo, and Ex Vivo Evaluation. AAPS PharmSciTech 2019, 20, 210. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors affecting liposomes particle size prepared by ethanol injection method. Res. Pharm. Sci. 2017, 12, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alharbi, K.S.; Afzal, M.; Ali, R.; Alshehri, S.; Alzarea, S.I.; Elmowafy, M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Jain, S.; Harde, H.; Indulkar, A.; Agrawal, A. Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 431–440. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal Delivery of Ondansetron Hydrochloride via Bilosomal Systems: In Vitro, Ex Vivo, and In Vivo Characterization Studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Kesarla, R.; Tank, T.; Vora, P.A.; Shah, T.; Parmar, S.; Omri, A. Preparation and evaluation of nanoparticles loaded ophthalmic in situ gel. Drug Deliv. 2016, 23, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Shukr, M.H.; Ismail, S.; El-Hossary, G.G.; El-Shazly, A.H. Design and evaluation of mucoadhesive in situ liposomal gel for sustained ocular delivery of travoprost using two steps factorial design. J. Drug Deliv. Sci. Technol. 2021, 61, 102333. [Google Scholar] [CrossRef]
- Kouchak, M.; Malekahmadi, M.; Bavarsad, N.; Malehi, A.S.; Andishmand, L. Dorzolamide nanoliposome as a long action ophthalmic delivery system in open angle glaucoma and ocular hypertension patients. Drug Dev. Ind. Pharm. 2018, 44, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Nagarsenker, M.; Londhe, V. Preparation and evaluation of a liposomal formulation of sodium cromoglicate. Int. J. Pharm. 2003, 251, 49–56. [Google Scholar] [CrossRef]
- Siepmann, J.; Siepmann, F.J. Mathematical modeling of drug dissolution. Int. J. Pharm. 2013, 453, 12–24. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Li, Y.; Wang, H.; Liu, D.; Yang, X.; Pan, W. A novel hydrogel with dual temperature and pH responsiveness based on a nanostructured lipid carrier as an ophthalmic delivery system: Enhanced trans-corneal permeability and bioavailability of nepafenac. New J. Chem. 2017, 41, 3920–3929. [Google Scholar] [CrossRef]
- Morsi, N.; Ibrahim, M.; Refai, H.; El Sorogy, H. Nanoemulsion-based electrolyte triggered in situ gel for ocular delivery of acetazolamide. Eur. J. Pharm. Sci. 2017, 104, 302–314. [Google Scholar] [CrossRef]
- Han, S.; Shen, J.-Q.; Gan, Y.; Geng, H.-M.; Zhang, X.-X.; Zhu, C.-L.; Gan, L. Novel vehicle based on cubosomes for ophthalmic delivery of flurbiprofen with low irritancy and high bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Saettone, M.F.; Chetoni, P.; Cerbai, R.; Mazzanti, G.; Braghiroli, L. Evaluation of ocular permeation enhancers: In vitro effects on corneal transport of four [beta]-blockers and in vitro/in vivo toxic activity. Int. J. Pharm. 1996, 142, 103–113. [Google Scholar] [CrossRef]
- Tavakoli, M.; Mahboobian, M.M.; Nouri, F.; Mohammadi, M. Studying the ophthalmic toxicity potential of developed ketoconazole loaded nanoemulsion in situ gel formulation for ophthalmic administration. Toxicol. Mech. Methods 2021, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, S.N.; Mahboobian, M.M. Besifloxacin-loaded ocular nanoemulsions: Design, formulation and efficacy evaluation. Drug Deliv. Transl. Res. 2022, 12, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of Metformin Hydrochloride Tailoring Bilosomes as an Effective Transdermal Nanocarrier. Int. J. Nanomed. 2022, 17, 1185–1201. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Burgalassi, S.; Tampucci, S.; Zucchetti, E.; Chipala, E.; Alany, R.G.; Al-Kinani, A.A.; Monti, D. A hybrid ocular delivery system of cyclosporine-A comprising nanomicelle-laden polymeric inserts with improved efficacy and tolerability. Biomater. Sci. 2021, 9, 8235–8248. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Jacob, S.; Al-Dhubiab, B.E.; Sreeharsha, N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Shinu, P.; Venugopala, K.N. Experimental design, formulation and in vivo evaluation of a novel topical in situ gel system to treat ocular infections. PLoS ONE 2021, 16, e0248857. [Google Scholar] [CrossRef]
- Upadhayay, P.; Kumar, M.; Pathak, K. Norfloxacin Loaded pH Triggered Nanoparticulate in-situ Gel for Extraocular Bacterial Infections: Optimization, Ocular Irritancy and Corneal Toxicity. Iran. J. Pharm. Res. 2016, 15, 3–22. [Google Scholar]
- Jain, P.; Jaiswal, C.P.; Mirza, M.A.; Anwer, K.; Iqbal, Z. Preparation of levofloxacin loaded in situ gel for sustained ocular delivery: In vitro and ex vivo evaluations. Drug Dev. Ind. Pharm. 2020, 46, 50–56. [Google Scholar] [CrossRef]
- Youssef, A.; Dudhipala, N.; Majumdar, S. Ciprofloxacin Loaded Nanostructured Lipid Carriers Incorporated into In-Situ Gels to Improve Management of Bacterial Endophthalmitis. Pharmaceutics 2020, 12, 572. [Google Scholar] [CrossRef]
- Vella, J.; Busuttil, F.; Bartolo, N.S.; Sammut, C.; Ferrito, V.; Serracino-Inglott, A.; Azzopardi, L.M.; LaFerla, G. A simple HPLC–UV method for the determination of ciprofloxacin in human plasma. J. Chromatogr. B 2015, 989, 80–85. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Nano-transfersomal ciprofloxacin loaded vesicles for non-invasive trans-tympanic ototopical delivery: In-vitro optimization, ex-vivo permeation studies, and in-vivo assessment. Int. J. Pharm. 2014, 472, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Marzok, S.; Ibrahim, M. Development of optimized mucoadhesive thermosensitive pluronic based in situ gel for controlled delivery of Latanoprost: Antiglaucoma efficacy and stability approaches. J. Drug Deliv. Sci. Technol. 2019, 53, 101134. [Google Scholar] [CrossRef]
- Kalweit, S.; Besoke, R.; Gerner, I.; Spielmann, H. A national validation project of alternative methods to the Draize rabbit eye test. Toxicol. Vitr. 1990, 4, 702–706. [Google Scholar] [CrossRef]








| Batch Code | Independent Variables | Independent Variables | |||||
|---|---|---|---|---|---|---|---|
| CHO (mg) | Span-60 (mg) | SDC (mg) | VS (nm) | EE (%) | |||
| Exp. Values | Pred. Values | Exp. Values | Pred. Values | ||||
| CIP-BLO1 | 10.00 | 40.00 | 20.00 | 148.3 ± 12.5 | 149.51 | 67.1 ± 2.7 | 66.71 |
| CIP-BLO2 | 30.00 | 40.00 | 20.00 | 259.6 ± 18.1 | 260.05 | 85.3 ± 2.1 | 85.30 |
| CIP-BLO3 | 10.00 | 60.00 | 20.00 | 113.3 ± 7.9 | 112.94 | 76.2 ± 3.1 | 76.11 |
| CIP-BLO4 | 30.00 | 60.00 | 20.00 | 191.7 ± 14.7 | 190.55 | 88.1 ± 1.9 | 89.41 |
| CIP-BLO5 | 10.00 | 50.00 | 15.00 | 149.6 ± 9.3 | 149.06 | 68.1 ± 1.4 | 68.40 |
| CIP-BLO6 | 30.00 | 50.00 | 15.00 | 239.5 ± 16.8 | 240.10 | 85.0 ± 2.7 | 85.69 |
| CIP-BLO7 | 10.00 | 50.00 | 25.00 | 120.8 ± 6.2 | 126.64 | 70.3 ± 2.3 | 70.41 |
| CIP-BLO8 | 30.00 | 50.00 | 25.00 | 223.1 ± 14.8 | 223.76 | 86.3 ± 2.8 | 85.01 |
| CIP-BLO9 | 20.00 | 40.00 | 15.00 | 233.0 ± 19.3 | 232.48 | 75.7 ± 1.8 | 75.75 |
| CIP-BLO10 | 20.00 | 60.00 | 15.00 | 161.0 ± 12.4 | 162.01 | 80.3 ± 3.1 | 80.04 |
| CIP-BLO11 | 20.00 | 40.00 | 25.00 | 196.6 ± 8.3 | 195.67 | 73.7 ± 2.4 | 73.95 |
| CIP-BLO12 | 20.00 | 60.00 | 25.00 | 159.5 ± 9.2 | 160.07 | 83.3 ± 3.1 | 83.17 |
| CIP-BLO13 | 20.00 | 50.00 | 20.00 | 170.6 ± 7.6 | 171.04 | 77.2 ± 2.6 | 77.59 |
| CIP-BLO14 | 20.00 | 50.00 | 20.00 | 170.0 ± 9.2 | 171.04 | 78.5 ± 2.9 | 77.59 |
| CIP-BLO15 | 20.00 | 50.00 | 20.00 | 172.2 ± 7.3 | 171.04 | 77.6 ± 2.4 | 77.59 |
| CIP-BLO16 | 20.00 | 50.00 | 20.00 | 170.3 ± 9.7 | 171.04 | 78.2 ± 2.3 | 77.59 |
| CIP-BLO17 | 20.00 | 50.00 | 20.00 | 171.9 ± 8.3 | 171.04 | 77.5 ± 3.2 | 77.59 |
| Source | Vesicle Size (nm) | Entrapment Efficiency (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sum of the Square | DF | Mean Square | F-Value | p-Value | Sum of the Square | DF | Mean Square | F-Value | p-Value | |
| Model | 25,397.74 | 9 | 2821.97 | 1937.80 | <0.0001 * | 625.84 | 9 | 69.54 | 514.71 | <0.0001 * |
| Residual | 10.19 | 7 | 1.46 | - | - | 0.95 | 7 | 0.14 | - | - |
| Lack of Fit | 6.31 | 3 | 2.10 | 2.17 | 0.2347 ** | 0.53 | 3 | 0.18 | 1.73 | 0.2984 ** |
| Pure Error | 3.88 | 4 | 0.97 | - | - | 0.41 | 4 | 0.10 | - | - |
| Total | 25,407.94 | 16 | - | - | - | 626.78 | 16 | - | - | - |
| Formulation Code | Clarity | % transmission | Gelling Strength | Viscosity (cP) | Drug Content (%) | ||
|---|---|---|---|---|---|---|---|
| Sol (5.4 ± 0.02) | Gel (STF, pH 7.4 ± 0.02) | Sol (5.4 ± 0.02) | Gel (STF, pH 7.4 ± 0.02) | ||||
| CIP-BLO-opt-IG1 | Transparent/Clear | 92.82 ± 2.36 | − | + | 10.7 8 ± 1.27 | 26.43 ± 3.86 | 94.56 ± 3.26 |
| CIP-BLO-opt-IG12 | Transparent/Clear | 94.52 ± 1.73 | − | ++ | 25.42 ± 2.42 | 145.85 ± 9.48 | 95.72 ± 2.84 |
| CIP-BLO-opt-IG13 | Transparent/Clear | 98.65 ± 1.92 | − | +++ | 55.43 ± 6.28 | 266.32 ± 9.78 | 98.92 ± 2.43 |
| CIP-BLO-opt-IG14 | Not Transparent | 91.15 ± 2.81 | + | ++++ | 107.43 ± 5.81 | 387.73 ± 15.54 | 99.31 ± 1.60 |
| CIP-BLO-opt-IG15 | Turbid | 81.83 ± 2.48 | + | ++++ | 128.51 ± 2.03 | 431.63 ± 14.23 | 99.11 ± 1.01 |
| Independent Variable | Dependent Variable | Goal | ||
|---|---|---|---|---|
| Name and Unit | Level | |||
| Lower (−1) | Upper (+1) | |||
| Cholesterol (CHO, mg) | 10 | 30 | Vesicle size (VS, nm) | Optimum |
| Surfactant (span 60, mg) | 40 | 60 | Entrapment efficiency (EE, %) | Maximum |
| Bile salt (SDC, mg) | 15 | 25 | − | − |
| Ingredients | Formulation Code | ||||
|---|---|---|---|---|---|
| CIP-BLO-opt-IG1 | CIP-BLO-opt-IG2 | CIP-BLO-opt-IG3 | CIP-BLO-opt-IG4 | CIP-BLO-opt-IG5 | |
| Carbopol 934P (% w/v) | 1.0 | 1.2 | 1.4 | 1.6 | 1.8 |
| HPMC K100M (% w/v) | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
| Benzalkonium Chloride (% v/v) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Sodium chloride (g) | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Distilled water (mL) | 100 | 100 | 100 | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687. https://doi.org/10.3390/gels8110687
Alsaidan OA, Zafar A, Yasir M, Alzarea SI, Alqinyah M, Khalid M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels. 2022; 8(11):687. https://doi.org/10.3390/gels8110687
Chicago/Turabian StyleAlsaidan, Omar Awad, Ameeduzzafar Zafar, Mohd Yasir, Sami I. Alzarea, Mohammed Alqinyah, and Mohammad Khalid. 2022. "Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study" Gels 8, no. 11: 687. https://doi.org/10.3390/gels8110687
APA StyleAlsaidan, O. A., Zafar, A., Yasir, M., Alzarea, S. I., Alqinyah, M., & Khalid, M. (2022). Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels, 8(11), 687. https://doi.org/10.3390/gels8110687