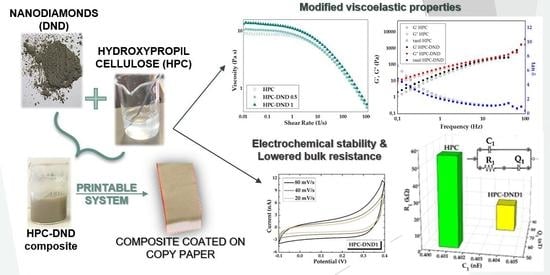
A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Structural Characterizaition
2.1.1. Detonation Nanodiamond (DND)
2.1.2. Nanocomposite Characterization
Rheological Measurements
2.2. NMR Investigation
2.3. Electrochemical and Charge Transport Properties Investigation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of the Polymeric Solution and DND Composite
4.2.2. Characterization Techniques
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Polino, G.; Scaramella, A.; Manca, V.; Palmieri, E.; Tamburri, E.; Orlanducci, S.; Brunetti, F. Nanodiamond-Based Separators for Supercapacitors Realized on Paper Substrates. Energy Technol. 2020, 8, 1901233. [Google Scholar] [CrossRef]
- Hasanin, M.; Mwafy, E.A.; Youssef, A.M. Electrical Properties of Conducting Tertiary Composite Based on Biopolymers and Polyaniline. J. Bio-Tribo-Corros. 2021, 7, 133. [Google Scholar] [CrossRef]
- Kasprzak, D.; Stępniak, I.; Galiński, M. Electrodes and Hydrogel Electrolytes Based on Cellulose: Fabrication and Characterization as EDLC Components. J. Solid State Electrochem. 2018, 22, 3035–3047. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Ma, G.; Ying, W.; Wang, A.; Huang, H.; Lei, Z. In Situ Synthesis of Polyaniline/Sodium Carboxymethyl Cellulose Nanorods for High-Performance Redox Supercapacitors. J. Power Sources 2012, 211, 40–45. [Google Scholar] [CrossRef]
- Palmieri, E.; Montaina, L.; Polino, G.; Bonomo, M.; Giordanengo, G.; Barolo, C.; Paradossi, G.; Brunetti, F.; Tamburri, E.; Orlanducci, S. Engineered Surface for High Performance Electrodes on Paper. Appl. Surf. Sci. 2023, 608, 155117. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, G.; Zhu, Y.; Cheng, W.; Cao, K.; Xu, G.; Zhao, D.; Yu, H. A Scalable Bacterial Cellulose Ionogel for Multisensory Electronic Skin. Research 2022, 2022, 9814767. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brevik, I.; Hamsan, M.H.; Brza, M.A.; Nofal, M.M.; Abdullah, A.M.; Rostam, S.; Al-Zangana, S.; Muzakir, S.K.; Kadir, M.F.Z. Compatible Solid Polymer Electrolyte Based on Methyl Cellulose for Energy Storage Application: Structural, Electrical, and Electrochemical Properties. Polymers 2020, 12, 2257. [Google Scholar] [CrossRef]
- Chai, M.N.; Isa, M.I.N.; Chai, M.N.; Isa, M.I.N. The Oleic Acid Composition Effect on the Carboxymethyl Cellulose Based Biopolymer Electrolyte. J. Cryst. Process Technol. 2013, 3, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Zugenmaier, P. Cellulose. In Crystalline Cellulose and Derivatives: Characterization and Structures; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2008; pp. 101–174. [Google Scholar]
- Majewicz, T.G.; Podlas, T.J. Cellulose Ethers. Kirk-Othmer Encycl. Chem. Technol. 2000, 5, 507–532. [Google Scholar] [CrossRef]
- Heinze, T.; El Seoud, O.; Koschella, A. Cellulose Derivatives. Synthesis, Structure, and Properties; Springer: Cham, Switzerland, 2018; pp. 293–296. [Google Scholar]
- Palmieri, E.; Cicero, C.; Orazi, N.; Mercuri, F.; Zammit, U.; Mazzuca, C.; Orlanducci, S. Nanodiamond Composites: A New Material for the Preservation of Parchment. J. Appl. Polym. Sci. 2022, 139, e52742. [Google Scholar] [CrossRef]
- Rincón-Iglesias, M.; Lizundia, E.; Lanceros-Méndez, S. Water-Soluble Cellulose Derivatives as Suitable Matrices for Multifunctional Materials. Biomacromolecules 2019, 20, 2786–2795. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Umemura, K.; Tahara, K.; Takeuchi, H. Formulation Design of Hydroxypropyl Cellulose Films for Use as Orally Disintegrating Dosage Forms. J. Drug Deliv. Sci. Technol. 2018, 46, 93–100. [Google Scholar] [CrossRef]
- Filip, D.; Macocinschi, D.; Zaltariov, M.F.; Ciubotaru, B.I.; Bargan, A.; Varganici, C.D.; Vasiliu, A.L.; Peptanariu, D.; Balan-Porcarasu, M.; Timofte-Zorila, M.M. Hydroxypropyl Cellulose/Pluronic-Based Composite Hydrogels as Biodegradable Mucoadhesive Scaffolds for Tissue Engineering. Gels 2022, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Than, Y.M.; Suriyarak, S.; Titapiwatanakun, V. Rheological Investigation of Hydroxypropyl Cellulose–Based Filaments for Material Extrusion 3D Printing. Polymers 2022, 14, 1108. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.; Karan, N.K.; Thomas, R.; Pradhan, D.K.; Katiyar, R.S. Observation of Ionic Transport and Ion-Coordinated Segmental Motions in Composite (Polymer-Salt-Clay) Solid Polymer Electrolyte. Ionics 2015, 21, 401–410. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Heydaripour, S.; Abdouss, M. Novel Carboxymethyl Cellulose Based Nanocomposite Membrane: Synthesis, Characterization and Application in Water Treatment. J. Environ. Manag. 2016, 166, 457–465. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Daliri Sousefi, M.; Ashori, A.; Ebrahimi, G. Preparation and Characterization of Sodium Carboxymethyl Cellulose/Silk Fibroin/Graphene Oxide Nanocomposite Films. Polym. Test. 2016, 52, 218–224. [Google Scholar] [CrossRef]
- Lizundia, E.; Urruchi, A.; Vilas, J.L.; León, L.M. Increased Functional Properties and Thermal Stability of Flexible Cellulose Nanocrystal/ZnO Films. Carbohydr. Polym. 2016, 136, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–Polymer Composites. Diam. Relat. Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Shenderova, O.; Tyler, T.; Cunningham, G.; Ray, M.; Walsh, J.; Casulli, M.; Hens, S.; McGuire, G.; Kuznetsov, V.; Lipa, S. Nanodiamond and Onion-like Carbon Polymer Nanocomposites. Diam. Relat. Mater. 2007, 16, 1213–1217. [Google Scholar] [CrossRef]
- Mochalin, V.; Shenderova, O.; Ho, D.; Gogotsi, Y. The Properties and Applications of Nanodiamonds. In Nano-Enabled Medical Applications; Routledge: London, UK, 2020; pp. 313–350. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-Caprolactone)-Based Electrospun Nano-Featured Substrate for Tissue Engineering Applications: A Review. Prog. Biomater. 2021, 10, 91–117. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Review on Conducting Polymer/Nanodiamond Nanocomposites: Essences and Functional Performance. J. Plast. Film Sheeting 2019, 35, 331–353. [Google Scholar] [CrossRef]
- Shkodich, V.; Temnikova, N.; Boyko, I.; Leicht, H.; Kraus, E.; Shkodich, N.; Stoyanov, O. Structural Properties of Nanocomposites Based on Resole-Type Phenol-Formaldehyde Oligomers and Detonation Nanodiamonds. J. Appl. Polym. Sci. 2020, 137, 43–46. [Google Scholar] [CrossRef]
- Hopper, A.P.; Dugan, J.M.; Gill, A.A.; Fox, O.J.L.; May, P.W.; Haycock, J.W.; Claeyssens, F. Amine Functionalized Nanodiamond Promotes Cellular Adhesion, Proliferation and Neurite Outgrowth. Biomed. Mater. 2014, 9, 045009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angjellari, M.; Tamburri, E.; Montaina, L.; Natali, M.; Passeri, D.; Rossi, M.; Terranova, M.L. Beyond the Concepts of Nanocomposite and 3D Printing: PVA and Nanodiamonds for Layer-by-Layer Additive Manufacturing. Mater. Des. 2017, 119, 12–21. [Google Scholar] [CrossRef]
- Matassa, R.; Orlanducci, S.; Reina, G.; Cassani, M.C.; Passeri, D.; Terranova, M.L.; Rossi, M. Structural and Morphological Peculiarities of Hybrid Au/Nanodiamond Engineered Nanostructures. Sci. Rep. 2016, 6, 31163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reina, G.; Gismondi, A.; Carcione, R.; Nanni, V.; Peruzzi, C.; Angjellari, M.; Chau, N.D.Q.; Canini, A.; Terranova, M.L.; Tamburri, E. Oxidized and Amino-Functionalized Nanodiamonds as Shuttle for Delivery of Plant Secondary Metabolites: Interplay between Chemical Affinity and Bioactivity. Appl. Surf. Sci. 2019, 470, 744–754. [Google Scholar] [CrossRef]
- Tamburri, E.; Guglielmotti, V.; Orlanducci, S.; Terranova, M.L.; Sordi, D.; Passeri, D.; Matassa, R.; Rossi, M. Nanodiamond-Mediated Crystallization in Fibers of PANI Nanocomposites Produced by Template-Free Polymerization: Conductive and Thermal Properties of the Fibrillar Networks. Polymer 2012, 53, 4045–4053. [Google Scholar] [CrossRef] [Green Version]
- Grizzuti, N.; Cavella, S.; Cicarelli, P. Transient and steadystate rheology of a liquid crystalline hydroxypropylcellulose solution. J. Rheol. 1990, 34, 1293–1310. [Google Scholar] [CrossRef]
- Passeri, D.; Biagioni, A.; Rossi, M.; Tamburri, E.; Terranova, M.L. Characterization of Polyaniline-Detonation Nanodiamond Nanocomposite Fibers by Atomic Force Microscopy Based Techniques. Eur. Polym. J. 2013, 49, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Passeri, D.; Tamburri, E.; Terranova, M.L.; Rossi, M. Polyaniline-Nanodiamond Fibers Resulting from the Self-Assembly of Nano-Fibrils: A Nanomechanical Study. Nanoscale 2015, 7, 14358–14367. [Google Scholar] [CrossRef] [PubMed]
- Tamburri, E.; Guglielmotti, V.; Matassa, R.; Orlanducci, S.; Gay, S.; Reina, G.; Terranova, M.L.; Passeri, D.; Rossi, M. Detonation Nanodiamonds Tailor the Structural Order of PEDOT Chains in Conductive Coating Layers of Hybrid Nanoparticles. J. Mater. Chem. C 2014, 2, 3703–3716. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Robertson, J. Raman Spectroscopy of Amorphous, Nanostructured, Diamondlike Carbon, and Nanodiamond. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2477–2512. [Google Scholar] [CrossRef]
- Popov, M.; Churkin, V.; Kirichenko, A.; Denisov, V.; Ovsyannikov, D.; Kulnitskiy, B.; Perezhogin, I.; Aksenenkov, V.; Blank, V. Raman Spectra and Bulk Modulus of Nanodiamond in a Size Interval of 2–5 Nm. Nanoscale Res. Lett. 2017, 12, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrari, A.C.; Robertson, J. Resonant Raman Spectroscopy of Disordered, Amorphous, and Diamondlike Carbon. Phys. Rev. B—Condens. Matter Mater. Phys. 2001, 64, 075414. [Google Scholar] [CrossRef] [Green Version]
- Mochalin, V.; Osswald, S.; Gogotsi, Y. Contribution of Functional Groups to the Raman Spectrum of Nanodiamond Powders. Chem. Mater. 2009, 21, 273–279. [Google Scholar] [CrossRef]
- Orlanducci, S.; Fiori, A.; Sessa, V.; Tamburri, E.; Toschi, F.; Terranova, M.L. Nanocrystalline Diamond Films Grown in Nitrogen Rich Atmosphere: Structural and Field Emission Properties. J. Nanosci. Nanotechnol. 2008, 8, 3228–3234. [Google Scholar] [CrossRef]
- Carcione, R.; Tamburri, E.; Bartali, R.; Speranza, G.; Micheli, V.; Pepponi, G.; Bellutti, P.; Terranova, M.L. On the Route to Produce Conductive Ni-Related Color Centers in CVD-Grown Diamond. Multifunct. Mater. 2019, 2, 035001. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.; Huang, L.; Maximov, M.Y.; Jin, M.; Zhang, Y.; Wang, X.; Zhou, G. Biomass-Derived Oxygen and Nitrogen Co-Doped Porous Carbon with Hierarchical Architecture as Sulfur Hosts for High-Performance Lithium/Sulfur Batteries. Nanomaterials 2017, 7, 402. [Google Scholar] [CrossRef]
- Andreoli, E.; Barron, A.R. CO2 Adsorption by Para-Nitroaniline Sulfuric Acid-Derived Porous Carbon Foam. C 2016, 2, 25. [Google Scholar] [CrossRef] [Green Version]
- Carcione, R.; Politi, S.; Iacob, E.; Potrich, C.; Lunelli, L.; Vanzetti, L.E.; Bartali, R.; Micheli, V.; Pepponi, G.; Terranova, M.L.; et al. Exploring a New Approach for Regenerative Medicine: Ti-Doped Polycrystalline Diamond Layers as Bioactive Platforms for Osteoblast-like Cells Growth. Appl. Surf. Sci. 2021, 540, 148334. [Google Scholar] [CrossRef]
- Politi, S.; Carcione, R.; Tamburri, E.; Matassa, R.; Lavecchia, T.; Angjellari, M.; Terranova, M.L. Graphene Platelets from Shungite Rock Modulate Electropolymerization and Charge Storage Mechanisms of Soft-Template Synthetized Polypyrrole-Based Nanocomposites. Sci. Rep. 2018, 8, 17045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente, A.T.; Araújo, A.; Mendes, M.J.; Nunes, D.; Oliveira, M.J.; Sanchez-Sobrado, O.; Ferreira, M.P.; Águas, H.; Fortunato, E.; Martins, R. Multifunctional Cellulose-Paper for Light Harvesting and Smart Sensing Applications. J. Mater. Chem. C 2018, 6, 3143–3181. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tseng, S.R.; Chen, C.Y.; Meng, H.F.; Chen, E.C.; Horng, S.F.; Hsu, C.S. Polymer Solar Cell by Blade Coating. Org. Electron. 2009, 10, 741–746. [Google Scholar] [CrossRef]
- Davard, F.; Dupuis, D. Blade Coating of Fabrics: Rheology and Fluid Penetration. Color. Technol. 2002, 118, 69–74. [Google Scholar] [CrossRef]
- Rajan, K.; Bocchini, S.; Chiappone, A.; Roppolo, I.; Perrone, D.; Bejtka, K.; Ricciardi, C.; Pirri, C.F.; Chiolerio, A. Spin-Coated Silver Nanocomposite Resistive Switching Devices. Microelectron. Eng. 2017, 168, 27–31. [Google Scholar] [CrossRef]
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. 6 Studies of Spin-Coated Polymer Films. Annu. Rep. Sect. C Phys. Chem. 2005, 101, 174–201. [Google Scholar] [CrossRef]
- Tok, A.I.Y.; Boey, F.Y.C.; Lam, Y.C. Non-Newtonian Fluid Flow Model for Ceramic Tape Casting. Mater. Sci. Eng. A 2000, 280, 282–288. [Google Scholar] [CrossRef]
- Cross, M.M. Rheology of Non-Newtonian Fluids: A New Flow Equation for Pseudoplastic Systems. J. Colloid Sci. 1965, 20, 417–437. [Google Scholar] [CrossRef]
- Marrion, A.R. (Ed.) The Chemistry and Physics of Coatings; Royal Society of Chemistry: London, UK, 2004. [Google Scholar]
- Macosko, C.W. Chapter 2. In Rheology. Principles, Measurements and Applications; VCH Publishers: New York, NY, USA, 1994; pp. 65–108. [Google Scholar]
- Macosko, C.W. Chapter 3. In Rheology. Principles, Measurements and Applications; VCH Publishers: New York, NY, USA, 1994; pp. 109–127. [Google Scholar]
- Osterhold, M. Rheological Methods for Characterising Modern Paint Systems. Prog. Org. Coat. 2000, 40, 131–137. [Google Scholar] [CrossRef]
- Navard, P.; Haudin, J.M. Rheology of Hydroxypropylcellulose Solutions. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 189–201. [Google Scholar] [CrossRef]
- Ivanov, M.; Shenderova, O. Nanodiamond-Based Nanolubricants for Motor Oils. Curr. Opin. Solid State Mater. Sci. 2017, 21, 17–24. [Google Scholar] [CrossRef]
- Ivanov, M.G.; Pavlyshko, S.V.; Ivanov, D.M.; Petrov, I.; Shenderova, O. Synergistic Compositions of Colloidal Nanodiamond as Lubricant-Additive. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2010, 28, 869. [Google Scholar] [CrossRef]
- Hsissou, R.; Bekhta, A.; Dagdag, O.; El Bachiri, A.; Rafik, M.; Elharfi, A. Rheological Properties of Composite Polymers and Hybrid Nanocomposites. Heliyon 2020, 6, e04187. [Google Scholar] [CrossRef] [PubMed]
- Giovino, M.; Pribyl, J.; Benicewicz, B.; Kumar, S.; Schadler, L. Linear Rheology of Polymer Nanocomposites with Polymer-Grafted Nanoparticles. Polymer 2017, 131, 104–110. [Google Scholar] [CrossRef]
- Tschoegl, N. The Phenomenological Theory of Linear Viscoelastic Behavior. An Introduction; Springer: Berlin, Germany, 1989; Chapter 11; pp. 508–536. [Google Scholar]
- Lapasin, R.; Pricl, S. Rheometry. In Rheology of Industrial Polysaccharides: Theory and Applications; Springer: Boston, MA, USA, 1995; pp. 498–578. [Google Scholar]
- McCrum, N.G.; Read, B.E.; Williams, G. Anelastic and Dielectric Effects in Polymeric Solids; Wiley: New York, NY, USA, 1967. [Google Scholar] [CrossRef]
- Galesso, D.; Finelli, I.; Paradossi, G.; Renier, D. Viscoelastic Properties and Elastic Recovery of HYADD®4 Hydrogel Compared to Crosslinked HA-Based Commercial Viscosupplements. Osteoarthr. Cartil. 2012, 20, S292. [Google Scholar] [CrossRef] [Green Version]
- Hassanabadi, H.M.; Wilhelm, M.; Rodrigue, D. A Rheological Criterion to Determine the Percolation Threshold in Polymer Nano-Composites. Rheol. Acta 2014, 53, 869–882. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Moldenaers, P.; Vermant, J. Assessment of the Dispersion Quality in Polymer Nanocomposites by Rheological Methods. Macromol. Mater. Eng. 2011, 296, 331–340. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Q. Linear Rheology of Nanofilled Polymers. J. Rheol. 2015, 59, 155–191. [Google Scholar] [CrossRef]
- Nicolay, K.; Braun, K.P.J.; De Graaf, R.A.; Dijkhuizen, R.M.; Kruiskamp, M.J. Diffusion NMR Spectroscopy. NMR Biomed. 2001, 14, 94–111. [Google Scholar] [CrossRef]
- Jones, J.A.; Wilkins, D.K.; Smith, L.J.; Dobson, C.M. Characterisation of Protein Unfolding by NMR Diffusion Measurements. J. Biomol. 1997, 10, 199–203. [Google Scholar] [CrossRef]
- Wilkins, D.K.; Grimshaw, S.B.; Receveur, V.; Dobson, C.M.; Jones, J.A.; Smith, L.J. Hydrodynamic Radii of Native and Denatured Proteins Measured by Pulse Field Gradient NMR Techniques. Biochemistry 1999, 38, 16424–16431. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, H.; Yegani, R.; Babaeipour, V. Study on the Reinforcing Effect of Nanodiamond Particles on the Mechanical, Thermal and Antibacterial Properties of Cellulose Acetate Membranes. Diam. Relat. Mater. 2016, 69, 166–176. [Google Scholar] [CrossRef]
- Postnov, V.N.; Mel’nikova, N.A.; Shul’meister, G.A.; Novikov, A.G.; Murin, I.V.; Zhukov, A.N. Nafion- and Aquivion-Based Nanocomposites Containing Detonation Nanodiamonds. Russ. J. Gen. Chem. 2017, 87, 2754–2755. [Google Scholar] [CrossRef]
- Gareeva, F.; Petrova, N.; Shenderova, O.; Zhukov, A. Electrokinetic Properties of Detonation Nanodiamond Aggregates in Aqueous KCl Solutions. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 440, 202–207. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Jerschow, A.; Müller, N.; Jerschow, A.; Müller, N. Suppression of Convection Artifacts in Stimulated-Echo Diffusion Experiments. Double-Stimulated-Echo Experiments. J. Magn. Reson. 1997, 125, 372–375. [Google Scholar] [CrossRef]






| Molecule | Relative Population | D [m2s−1] | Rh [nm] | |
|---|---|---|---|---|
| HPC 4% | dioxane | 1.14 × 10−9 | 0.2 a | |
| 1 | 0.2 | 1.27 × 10−10 | 2 | |
| 2 | 0.4 | 1.60 × 10−11 | 15 | |
| 3 | 0.4 | 2.10 × 10−12 | 115 | |
| HPC 4% DND 0.25% | dioxane | 1.04 × 10−9 | 0.2 a | |
| 1 | 0.3 | 2.74 × 10−10 | 0.8 | |
| 2 | 0.4 | 1.84 × 10−11 | 12 | |
| 3 | 0.3 | 9.24 × 10−13 | 238 | |
| HPC 4% DND 0.5% | dioxane | 9.35 × 10−10 | 0.2 a | |
| 1 | 0.3 | 2.61 × 10−10 | 0.8 | |
| 2 | 0.3 | 1.30 × 10−11 | 15 | |
| 3 | 0.4 | 9.23 × 10−13 | 215 |
| R1 | Q1 | C1 | |
|---|---|---|---|
| HPC | 56.29 kΩ | 0.058 µT | 0.401 nF |
| 0.895 Φ | |||
| HPC-DND 1 | 17.57 kΩ | 0.081 µT | 0.404 nF |
| 0.869 Φ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmieri, E.; Pescosolido, F.; Montaina, L.; Carcione, R.; Petrella, G.; Cicero, D.O.; Tamburri, E.; Battistoni, S.; Orlanducci, S. A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications. Gels 2022, 8, 783. https://doi.org/10.3390/gels8120783
Palmieri E, Pescosolido F, Montaina L, Carcione R, Petrella G, Cicero DO, Tamburri E, Battistoni S, Orlanducci S. A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications. Gels. 2022; 8(12):783. https://doi.org/10.3390/gels8120783
Chicago/Turabian StylePalmieri, Elena, Francesca Pescosolido, Luca Montaina, Rocco Carcione, Greta Petrella, Daniel Oscar Cicero, Emanuela Tamburri, Silvia Battistoni, and Silvia Orlanducci. 2022. "A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications" Gels 8, no. 12: 783. https://doi.org/10.3390/gels8120783
APA StylePalmieri, E., Pescosolido, F., Montaina, L., Carcione, R., Petrella, G., Cicero, D. O., Tamburri, E., Battistoni, S., & Orlanducci, S. (2022). A Sustainable Hydroxypropyl Cellulose-Nanodiamond Composite for Flexible Electronic Applications. Gels, 8(12), 783. https://doi.org/10.3390/gels8120783