Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review
Abstract
:1. Introduction
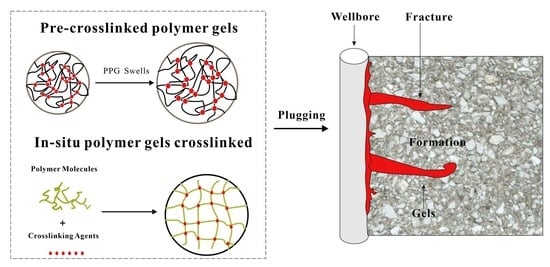
2. Types of Polymer Gels Used for Oil-Gas Drilling and Production
2.1. In Situ Polymer Gels Crosslinked
2.2. Pre-Crosslinked Polymer Gels
3. Improving Temperature Resistance of Polymer Gels
4. Improving Salt Resistance of Polymer Gels
5. Improving Rheological and Mechanical Properties of Polymer Gels
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, D.Y.; Bai, B.J.; Hou, J.R. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Sharma, P.; Kudapa, V.K. Study on the effect of cross-linked gel polymer on water shutoff in oil wellbores. Mater. Today Proc. 2022, 48, 1103–1106. [Google Scholar] [CrossRef]
- Leng, J.Y.; Wei, M.Z.; Bai, B.J. Review of transport mechanisms and numerical simulation studies of preformed particle gel for conformance control. J. Pet. Sci. Eng. 2021, 206, 109051. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Hussein, I.A. Polymer systems for water shutoff and profile modification: A review over the last decade. SPE J. 2014, 19, 135–149. [Google Scholar] [CrossRef]
- Kang, W.L.; Wang, J.Q.; Ye, Z.Q.; Gu, G.J.; Li, W.M.; Yang, H.B.; Li, Z.; Xu, H.X.; Lv, Z.Q.; Sarsenbekuly, B. Study on preparation and plugging effect of sawdust gel particle in fractured reservoir. J. Pet. Sci. Eng. 2022, 212, 110358. [Google Scholar] [CrossRef]
- Zhou, R.N.; Zhang, D.; Wei, J.G. Experiment on the profile control effect of different strength gel systems in heterogeneous reservoir. Energy Rep. 2021, 7, 6023–6030. [Google Scholar] [CrossRef]
- Hashmat, M.D.; Sultan, A.S.; Rahman, S.; Hussain, S.M.S. Crosslinked polymeric gels as loss circulation materials: An experimental study. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 25–28 April 2016. [Google Scholar]
- Magzoub, M.I.; Salehi, S.; Hussein, I.A.; Nasser, M.S. Loss circulation in drilling and well construction: The significance of applications of crosslinked polymers in wellbore strengthening: A review. J. Pet. Sci. Eng. 2020, 185, 106653. [Google Scholar] [CrossRef]
- Targac, G.; Gallo, C.; Smith, D.; Huang, C.K.; Autry, S.; Peirce, J.; Li, B. Case history of conformance solutions for west sak wormhole/void space conduit with a new reassembling pre-formed particle gel RPPG. In Proceedings of the SPE Annual Technical Conference and Exhibition, Virtual, 26–29 October 2020. [Google Scholar]
- Bai, B.; Wei, M.; Liu, Y. Field and lab experience with a successful preformed particle gel conformance control technology. In Proceedings of the SPE Production and Operations Symposium, Oklahoma City, OK, USA, 23–26 March 2013. [Google Scholar]
- Deolarte, C.; Vasquez, J.; Soriano, E.; Santillan, A. Successful combination of an organically crosslinked polymer system and a rigid-setting material for conformance control in Mexico. SPE Prod. Oper. 2009, 24, 522–529. [Google Scholar] [CrossRef]
- Esfahlan, M.S.; Khodapanah, E.; Tabatabaei-Nezhad, S.A. Comprehensive review on the research and field application of preformed particle gel conformance control technology. J. Pet. Sci. Eng. 2021, 202, 108440. [Google Scholar]
- Cui, C.X.; Zhou, Z.J.; He, Z. Enhance oil recovery in low permeability reservoirs: Optimization and evaluation of ultra-high molecular weight HPAM / phenolic weak gel system. J. Pet. Sci. Eng. 2020, 195, 107908. [Google Scholar] [CrossRef]
- Koh, J.K.; Lai, C.W.; Johan, M.R.; Gan, S.S.; Chua, W.W. Recent advances of modified polyacrylamide in drilling technology. J. Pet. Sci. Eng. 2022, 215, 110566. [Google Scholar] [CrossRef]
- Zhang, G.C.; Chen, L.F.; Ge, J.J.; Jiang, P.; Zhu, X.M. Experimental research of syneresis mechanism of HPAM/Cr3+ gel. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 96–103. [Google Scholar] [CrossRef]
- Sun, X.D.; Bai, B.J.; Alhuraishawy, A.K.; Zhu, D.Y. Understanding the plugging performance of HPAM-CR(III) Polymer gel for CO2 conformance control. SPE J. 2021, 26, 3109–3118. [Google Scholar] [CrossRef]
- Wang, J.; AlSofi, A.M.; AlBoqmi, A.M. Development and evaluation of gel-based conformance control for a high salinity and high temperature carbonate. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 21–23 March 2016. [Google Scholar]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zhou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2021, 202, 108416. [Google Scholar] [CrossRef]
- Durán-Valencia, C.; Bai, B.; Reyes, H.; Fajardo-López, R.; Barragán-Aroche, F.; López-Ramírez, S. Development of enhanced nanocomposite preformed particle gels for conformance control in high-temperature and high-salinity oil reservoirs. Polym. J. 2014, 46, 277–284. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, C.S.; Sang, H.B.; Zhao, Q. Mechanism Study of the Cross-Linking Reaction of Hydrolyzed Polyacrylamide/Ac3Cr in Formation Water. Energy Fuels 2015, 29, 4701–4710. [Google Scholar] [CrossRef]
- Tessarolli, F.G.C.; Souza, S.T.S.; Gomes, A.S.; Mansur, C.R.E. Gelation kinetics of hydrogels based on acrylamide–AMPS–NVP terpolymer, bentonite, and polyethylenimine for conformance control of oil reservoirs. Gels 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An experimental investigation of silica nanoparticles effect on the rheological behavior of polyacrylamide solution to enhance heavy oil recovery. Pet. Sci. Technol. 2013, 31, 500–508. [Google Scholar] [CrossRef]
- Amir, Z.; Said, I.M.; Jan, B.M. In situ organically cross-linked polymer gel for high-temperature reservoir conformance control: A review. Polym. Adv. Technol. 2019, 30, 13–39. [Google Scholar] [CrossRef] [Green Version]
- Pan, G.; Chen, J.; Zhang, C.; Liu, D.; Wu, J.; Li, H.; Fang, Z.; Qu, J.; Zhang, J. Combined technology of weak gel flooding assisting thermal huff and puff enhances oil recovery for offshore heavy oil field. In Proceedings of the SPE Annual Technical Conference and Exhibition, Dubai, United Arab Emirates, 26–28 September 2016. [Google Scholar]
- Zhang, X.; Zhang, S.; Li, L.; Wu, R.; Liu, D.; Wu, J.; Wu, W. High-temperature-resistant polymer gel system with metal-organic mixed cross-linking agents. J. Appl. Polym. Sci. 2015, 132, 1–8. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Sarsenbekuly, B.; Zhang, M.; Jiang, H.; Kang, W.; Aidarova, S. The advances of organic chromium based polymer gels and their application in improved oil recovery. Adv. Colloid Interface Sci. 2020, 282, 102214. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Kang, X.; Lashari, Z.A.; Li, Z.; Zhou, B.; Yang, H.; Sarsenbekuly, B.; Aidarova, S. Progress of polymer gels for conformance control in oil field. Adv. Colloid Interface Sci. 2021, 289, 102363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, C.; Wang, K.; Zhao, M.; Zhao, G.; Yang, S.; Yan, Z.; You, Q. New insights into the hydroquinone (HQ)-hexamethylenetetramine (HMTA) gel system for water shut-off treatment in high temperature reservoirs. J. Ind. Eng. Chem. 2016, 35, 20–28. [Google Scholar] [CrossRef]
- Gakhar, K.; Lane, R.H. Low extrusion pressure polymer gel for water shutoff in narrow aperture fractures in tight and shale gas and oil reservoirs. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012. [Google Scholar]
- Zhao, G.; Dai, C.; Chen, A.; Yan, Z.; Zhao, M. Experimental study and application of gels formed by nonionic polyacrylamide and phenolic resin for in-depth profile control. J. Pet. Sci. Eng. 2015, 135, 552–560. [Google Scholar] [CrossRef]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelation kinetics of PAM/PEI system. J. Therm. Anal. Calorim. 2014, 116, 1409–1415. [Google Scholar]
- Xie, B.Q.; Ma, J.; Wang, Y.; Tchameni, A.P.; Luo, M.W.; Wen, J.T. Enhanced hydrophobically modified polyacrylamide gel for lost circulation treatment in high temperature drilling. J. Mol. Liq. 2021, 325, 115155. [Google Scholar] [CrossRef]
- Wang, B.; Sun, J.S.; Lv, K.H.; Shen, F.; Bai, Y.R. Effects of a crosslinking agent on a supramolecular gel to control lost circulation. N. J. Chem. 2021, 45, 7089–7095. [Google Scholar] [CrossRef]
- Nie, X.; Luo, P.; Wang, P.; Zhang, X.; Yang, L. Rheology of a new gel used for severe lost circulation control. In Proceedings of the International Oil and Gas Conference and Exhibition in China, Beijing, China, 8–10 June 2010. [Google Scholar]
- Jiang, Q.; Jiang, G.; Wang, C.; Zhu, Q.; Yang, L.; Wang, L.; Zhang, X.; Liu, C. A new high-temperature shear-tolerant supramolecular viscoelastic fracturing fluid. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference, Singapore, 22–24 August 2016. [Google Scholar]
- Imqam, A.; Bai, B.J. Optimizing the strength and size of preformed particle gels for better conformance control treatment. Fuel 2015, 148, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Dai, C.L.; Liu, Y.F.; Zou, C.W.; You, Q.; Yang, S.; Zhao, M.W.; Zhang, G.; Wu, Y.; Sun, Y. Investigation on matching relationship between dispersed particle gel (DPG) and reservoir pore-throats for in-depth profile control. Fuel 2017, 207, 109–120. [Google Scholar] [CrossRef]
- Bai, B.J.; Li, L.X.; Liu, Y.Z.; Liu, H.; Wang, Z.G.; You, C.M. Preformed particle gel for conformance control: Factors affecting its properties and applications. SPE Reserv. Eval. Eng. 2007, 10, 415–422. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.J.; Zhou, J.; Yin, M.F. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Lenji, M.A.; Haghshenasfard, M.; Sefti, M.V.; Salehi, M.B.; Heidari, A. Experimental study of swelling and rheological behavior of preformed particle gel used in water shutoff treatment. J. Pet. Sci. Eng. 2018, 169, 739–747. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Liu, Y.; Tongwa, P. Thermo-dissoluble polymer for in-depth mobility control. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. [Google Scholar]
- Pu, J.; Bai, B.; Alhuraishawy, A.; Schuman, T.; Chen, Y.; Sun, X. A recrosslinkable preformed particle gel for conformance control in heterogeneous reservoirs containing linear-flow features. SPE J. 2019, 24, 1714–1725. [Google Scholar] [CrossRef]
- You, Q.; Tang, Y.; Dai, C.; Shuler, P.; Lu, Z.; Zhao, F. Research on a new profile control agent: Dispersed particle gel. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–20 July 2011. [Google Scholar]
- Zhao, G.; You, Q.; Tao, J.P.; Gu, C.L.; Aziz, H.; Ma, L.P.; Dai, C.L. Preparation and application of a novel phenolic resin dispersed particle gel for in-depth profile control in low permeability reservoirs. J. Pet. Sci. Eng. 2018, 161, 703–714. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ramhormozi, M.Z.; Wood, D.A.; Ashena, R. Reinforcement of oil and gas wellbore cements with a methyl methacrylate/carbon-nanotube polymer nanocomposite additive. Cem. Concr. Compos. 2020, 114, 103763. [Google Scholar] [CrossRef]
- Dai, C.L.; Chen, W.X.; You, Q.; Wang, H.; Zhe, Y.; He, L.; Jiao, B.L.; Wu, Y.N. A novel strengthened dispersed particle gel for enhanced oil recovery application. J. Ind. Eng. Chem. 2016, 41, 175–182. [Google Scholar] [CrossRef]
- Yao, C.; Xu, X.; Wang, D.; Lei, G.; Xue, S.; Hou, J.; Cathles, L.M.; Steenhuis, T.S. Research and application of micron-size polyacrylamide elastic microspheres as a smart sweep improvement and profile modification agent. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]
- Zhao, S.; Pu, W.F.; Wei, B.; Xu, X.G. A comprehensive investigation of polymer microspheres (PMs) migration in porous media: EOR implication. Fuel 2019, 235, 249–258. [Google Scholar] [CrossRef]
- Abdulbaki, M.; Huh, C.; Sepehrnoori, K.; Delshad, M.; Varavei, A. A critical review on use of polymer microgels for conformance control purposes. J. Pet. Sci. Eng. 2014, 122, 741–753. [Google Scholar] [CrossRef]
- Du, D.J.; Pu, W.F.; Zhang, S.; Jin, F.Y.; Wang, S.K.; Ren, F. Preparation and migration study of graphene oxide-grafted polymeric microspheres: EOR implications. J. Pet. Sci. Eng. 2020, 192, 107286. [Google Scholar] [CrossRef]
- Liu, L.; Gou, S.H.; Fang, S.W.; He, Y.; Tang, L. Organic-inorganic microspheres of temperature-controlled size for profile control. J. Mol. Liq. 2020, 317, 113993. [Google Scholar] [CrossRef]
- Juárez, J.L.; Rodriguez, M.R.; Montes, J.; Trujillo, F.D.; Monzòn, J.; Dupuis, G.; Gaillard, N. Conformance Gel Design for High Temperature Reservoirs. In Proceedings of the SPE Europec, Virtual, 1–3 December 2020. [Google Scholar]
- El-Karsani, K.S.M.; Al-Muntasheri, G.A.; Sultan, A.S.; Hussein, I.A. Gelation of a water-shutoff gel at high pressure and high temperature: Rheological investigation. SPE J. 2015, 20, 1103–1112. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Du, K.; Liu, H. Development of a new high-temperature and high-strength polymer gel for plugging fractured reservoirs. Upstream Oil Gas Technol. 2020, 5, 100014. [Google Scholar] [CrossRef]
- Long, Y.; Yu, B.; Zhu, C. Conformance improvement for ultra-high-temperature reservoir: A comparative study between hydrostable and conventional preformed particle gel. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 12–15 November 2018. [Google Scholar]
- Chen, L.; Zhang, G.; Ge, J.; Jiang, P.; Zhu, X.; Lin, Y.; Han, S. A novel thermal-resistance and salt-tolerance gel with low-concentration crosslinkers for water shutoff in Tahe oilfield. In Proceedings of the SPE Asia Pacific Unconventional Resources Conference and Exhibition, Brisbane, Australia, 9–11 November 2015. [Google Scholar]
- Zhu, D.; Hou, J.; Wei, Q.; Chen, Y.; Peng, K. Development of a high-temperature resistant polymer gel system for conformance control in jidong oilfield. SPE Reserv. Eval. Eng. 2019, 22, 100–109. [Google Scholar] [CrossRef]
- Zhang, S.L.; Guo, J.X.; Gu, Y.; Zhao, Q.; Yang, R.J.; Yang, Y.Q. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Chen, L.F.; Zhang, G.C.; Ge, J.J.; Jiang, P.; Zhu, X.M.; Ran, Y.L.; Han, S.X. Ultrastable Hydrogel for Enhanced Oil Recovery Based on Double-Groups Cross-Linking. Energy Fuels 2015, 29, 7196–7203. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Katbab, A.A.; Nabavizadeh, J.; Tabasi, R.Y.; Nejad, M.H. Preparation and characterization of nanocomposite hydrogels based on polyacrylamide for enhanced oil recovery applications. J. Appl. Polym. Sci. 2006, 100, 2096–2103. [Google Scholar] [CrossRef]
- Almoshin, A.M.; Alsharaeh, E.; Fathima, A.; Bataweel, M. A novel polymer nanocomposite graphene based gel for high temperature water shutoff applications. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Jia, H.; Niu, C.C.; Yang, X.Y. Improved understanding nanocomposite gel working mechanisms: From laboratory investigation to wellbore plugging application. J. Pet. Sci. Eng. 2020, 191, 107214. [Google Scholar] [CrossRef]
- Patil, P.; Kalgaonkar, R. Environmentally acceptable compositions comprising nanomaterials for plugging and sealing subterranean formations. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Sarsenbekuly, B.; Kang, W.L.; Fan, H.M.; Yang, H.B.; Dai, C.L.; Zhao, B.; Aidarova, S.B. Study of salt tolerance and temperature resistance of a hydrophobically modified polyacrylamide based novel functional polymer for EOR. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 91–97. [Google Scholar] [CrossRef]
- Fang, J.C.; Zhang, X.; He, L.; Zhao, G.; Dai, C.L. Experimental research of hydroquinone (HQ)/hexamethylene tetramine (HMTA) gel for water plugging treatments in high-temperature and high-salinity reservoirs. J. Appl. Polym. Sci. 2017, 134, 1–9. [Google Scholar] [CrossRef]
- Luo, W.; Dong, H.; Li, W.; Lin, Q.; Jian, F. Synthesis and property evaluation of a salt- and alkali-resistant star-polymer. Pet. Explor. Dev. 2010, 37, 477–482. [Google Scholar]
- Cai, H.; Luo, W.; Ma, D.; Zhou, X.; Fan, J.; Li, J.; Sun, J.; Zhang, Y. Experimental study and pilot test of combined gel treatment and surfactant imbibition technology in high temperature, high salinity reservoir. In Proceedings of the SPE Oklahoma City Oil and Gas Symposium, Oklahoma City, OK, USA, 27–31 March 2017. [Google Scholar]
- Yang, H.B.; Iqbal, M.W.; Lashari, Z.A.; Cao, C.X.; Tang, X.C.; Kang, W.L. Experimental research on amphiphilic polymer/organic chromium gel for high salinity reservoirs. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123900. [Google Scholar] [CrossRef]
- Saghafi, H.R.; Naderifar, A.; Gerami, S.; Emadi, M.A. Improvement in thermo-chemical stability of nanocomposite preformed particle gels for conformance control in harsh oil reservoir conditions. Can. J. Chem. Eng. 2016, 94, 1880–1890. [Google Scholar] [CrossRef]
- Pu, J.Y.; Geng, J.M.; Han, P.; Bai, B.J. Preparation and salt-insensitive behavior study of swellable, Cr3+-embedded microgels for water management. J. Mol. Liq. 2019, 273, 551–558. [Google Scholar] [CrossRef]
- Aldhaheri, M.; Wei, M.; Zhang, N.; Bai, B. A novel survey of bulk gel treatment designs in injection wells—Part 1: Gel strength. In Proceedings of the Annual Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2018. [Google Scholar]
- Tessarolli, F.G.C.; Souza, S.T.S.; Gomes, A.S.; Mansur, C.R.E. Influence of polymer structure on the gelation kinetics and gel strength of acrylamide-based copolymers, bentonite and polyethylenimine systems for conformance control of oil reservoirs. J. Appl. Polym. Sci. 2019, 136, 47556. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Wang, L.; Qu, S.; Wu, W.; Ji, R.; Luo, Y.; Yang, H. Nano-silica hybrid polyacrylamide/polyethylenimine gel for enhanced oil recovery at harsh conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127898. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H. Using DSC technique to investigate the non-isothermal gelation kinetics of the multi-crosslinked Chromium acetate (Cr3+)-Polyethyleneimine (PEI)-Polymer gel sealant. J. Pet. Sci. Eng. 2018, 165, 105–113. [Google Scholar] [CrossRef]
- Zhang, L.; Pu, C.S.; Cui, S.X.; Nasir, K.; Liu, Y. Experimental Study on a New Type of Water Shutoff Agent Used in Fractured Low Permeability Reservoir. J. Energy Resour. Technol. Trans. Asme 2017, 139, 1–9. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Hou, J.R.; Li, C.H. Water shut off in a horizontal well: Lab experiments with starch graft copolymer agent. J. Pet. Sci. Eng. 2013, 108, 230–238. [Google Scholar]
- Zhao, F.; Xu, T.; Hou, J.; Song, L.; Lu, G.; Feng, H. The applicable limits of the high strength gel system to the tight sandstone fractured reservoir in CO2 flooding. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 15–18 July 2019. [Google Scholar]
- Tongwa, P.; Nygaard, R.; Bai, B.J. Evaluation of a nanocomposite hydrogel for water shut-off in enhanced oil recovery applications: Design, synthesis, and characterization. J. Appl. Polym. Sci. 2013, 128, 787–794. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Khoshmardan, M.A. A hybrid nanocomposite of poly (styrene-methyl methacrylate-acrylic acid)/clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 33. [Google Scholar] [CrossRef]
- Mohamadian, N.; Ghorbani, H.; Wood, D.A.; Hormozi, H.K. Rheological and filtration characteristics of drilling fluids enhanced by nanoparticles with selected additives: An experimental study. Adv. Geo-Energy Res. 2018, 2, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Adewunmi, A.A.; Ismail, S.; Sultan, A.S. Crosslinked Polyacrylamide Composite Hydrogels Impregnated with Fly Ash: Synthesis, Characterization and Their Application as Fractures Sealant for High Water Producing Zones in Oil and Gas Wells. J. Polym. Environ. 2018, 26, 3294–3306. [Google Scholar] [CrossRef]
- Jia, H.; Chen, H.; Zhao, J.Z. Development of a highly elastic composite gel through novel intercalated crosslinking method for wellbore temporary plugging in high-temperature reservoirs. SPE J. 2020, 25, 2853–2866. [Google Scholar] [CrossRef]















| Strategy | Principle | Measure | Molecular Structure |
|---|---|---|---|
| Optimize polymer molecular structure | Introduce functional monomers (large side groups/rigid side groups, and hydrolysis-resistant/inhibiting amide monomers) | Introduce functional monomers and terpolymers (e.g., NVP and AMPS) |  (NVP) (NVP)  (AMPS) (AMPS)  (AM-AMPS-NVP) copolymer (AM-AMPS-NVP) copolymer |
| Select the appropriate type of chemical crosslinker | Use crosslinker with a cyclic structure to form temperature-resistant chemical bonds | Phenol/formaldehyde crosslinking, HQ/HMTA crosslinking system, PEI (PEI) |  (Phenol) (Phenol)  (catechol) (catechol)  (Formaldehyde) (Formaldehyde)  (Resorcinol) (Resorcinol)  (HQ) (HQ)  (HMTA) (HMTA)  (PEI) (PEI) |
| Optimize the gel network structure | Form an interpenetrating network structure in the gel to densify the 3D network of the gel | Introduce nanomaterials (e.g., nano-montmorillonite, nano-silica), use composite crosslinkers |  (Nano-silica) (Nano-silica) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, S.; Sun, J.; Lv, K.; Zhang, Q.; Yang, J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels 2022, 8, 386. https://doi.org/10.3390/gels8060386
Lei S, Sun J, Lv K, Zhang Q, Yang J. Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels. 2022; 8(6):386. https://doi.org/10.3390/gels8060386
Chicago/Turabian StyleLei, Shaofei, Jinsheng Sun, Kaihe Lv, Qitao Zhang, and Jingbin Yang. 2022. "Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review" Gels 8, no. 6: 386. https://doi.org/10.3390/gels8060386
APA StyleLei, S., Sun, J., Lv, K., Zhang, Q., & Yang, J. (2022). Types and Performances of Polymer Gels for Oil-Gas Drilling and Production: A Review. Gels, 8(6), 386. https://doi.org/10.3390/gels8060386