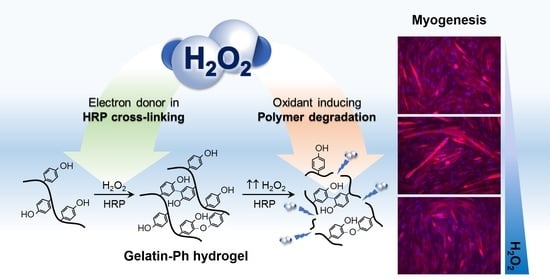
Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelatin-Ph Hydrogel Characterisation
2.2. Myoblasts Viability
2.3. Myoblasts Adhesion
2.4. Myoblasts Differentiation
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Gelatin-Ph Preparation
4.3. Scanning Electron Microscope Observation
4.4. Gelation Time Measurement
4.5. Mechanical Property Measurement
4.6. Enzymatic Degradation
4.7. Molecular Weight Measurement
4.8. Cell Culture
4.9. Cell Viability and Adhesion Analysis
4.10. Cell Differentiation Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.; Wang, Z.M.; Heymsfield, S.B.; Baumgartner, R.N.; Gallagher, D. Total-body skeletal muscle mass: Estimation by a new dual-energy X-ray absorptiometry method. Am. J. Clin. Nutr. 2002, 76, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Gumpenberger, M.; Wessner, B. Skeletal Muscle Extracellular Matrix—What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review. Front. Physiol. 2020, 11, 253. [Google Scholar] [CrossRef] [Green Version]
- Alarcin, E.; Bal-öztürk, A.; Avci, H.; Ghorbanpoor, H.; Guzel, F.D.; Akpek, A.; Yesiltas, G.; Canak-ipek, T.; Avci-adali, M. Current strategies for the regeneration of skeletal muscle tissue. Int. J. Mol. Sci. 2021, 22, 5929. [Google Scholar] [CrossRef]
- Rossi, C.A.; Pozzobon, M.; De Coppi, P. Advances in musculoskeletal tissue engineering: Moving towards therapy. Organogenesis 2010, 6, 167–172. [Google Scholar] [CrossRef] [Green Version]
- Maleiner, B.; Tomasch, J.; Heher, P.; Spadiut, O.; Rünzler, D.; Fuchs, C. The importance of biophysical and biochemical stimuli in dynamic skeletal muscle models. Front. Physiol. 2018, 9, 1130. [Google Scholar] [CrossRef]
- Narasimhan, B.N.; Horrocks, M.S.; Malmström, J. Hydrogels with Tunable Physical Cues and Their Emerging Roles in Studies of Cellular Mechanotransduction. Adv. NanoBiomed Res. 2021, 1, 2100059. [Google Scholar] [CrossRef]
- Zahari, N.K.; Idrus, R.B.H.; Chowdhury, S.R. Laminin-coated poly(Methyl methacrylate) (PMMA) nanofiber scaffold facilitates the enrichment of skeletal muscle myoblast population. Int. J. Mol. Sci. 2017, 18, 2242. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, C.; Hong, S.W.; Oh, J.W.; Han, D.W. Cell-adhesive matrices composed of RGD peptide-displaying M13 bacteriophage/poly(lactic-co-glycolic acid) nanofibers beneficial to myoblast differentiation. J. Nanosci. Nanotechnol. 2015, 15, 7907–7912. [Google Scholar] [CrossRef]
- Kumar, S.; Parekh, S.H. Linking graphene-based material physicochemical properties with molecular adsorption, structure and cell fate. Commun. Chem. 2020, 3, 8. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.C.; Lee, J.H.; Jin, L.; Kim, M.J.; Kim, Y.J.; Hyun, J.K.; Jung, T.G.; Hong, S.W.; Han, D.W. Stimulated myoblast differentiation on graphene oxide-impregnated PLGA-collagen hybrid fibre matrices matrices. J. Nanobiotechnol. 2015, 13, 21. [Google Scholar] [CrossRef] [Green Version]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Hayashi, K.; Matsuda, M.; Mitake, N.; Nakahata, M.; Munding, N.; Harada, A.; Kaufmann, S.; Takashima, Y.; Tanaka, M. One-Step Synthesis of Gelatin-Conjugated Supramolecular Hydrogels for Dynamic Regulation of Adhesion Contact and Morphology of Myoblasts. ACS Appl. Polym. Mater. 2022, 4, 2595–2603. [Google Scholar] [CrossRef]
- Denes, L.T.; Riley, L.A.; Mijares, J.R.; Arboleda, J.D.; McKee, K.; Esser, K.A.; Wang, E.T. Culturing C2C12 myotubes on micromolded gelatin hydrogels accelerates myotube maturation. Skelet. Muscle 2019, 9, 17. [Google Scholar] [CrossRef] [Green Version]
- Du, W.; Hong, S.; Scapin, G.; Goulard, M.; Shah, D.I. Directed Collective Cell Migration Using Three-Dimensional Bioprinted Micropatterns on Thermoresponsive Surfaces for Myotube Formation. ACS Biomater. Sci. Eng. 2019, 5, 3935–3943. [Google Scholar] [CrossRef]
- Mubarok, W.; Qu, Y.; Sakai, S. Influence of Hydrogen Peroxide-Mediated Cross-Linking and Degradation on Cell-Adhesive Gelatin Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4184–4190. [Google Scholar] [CrossRef]
- Ren, K.; He, C.; Xiao, C.; Li, G.; Chen, X. Injectable glycopolypeptide hydrogels as biomimetic scaffolds forcartilage tissue engineering. Biomaterials 2015, 51, 238–249. [Google Scholar] [CrossRef]
- Mubarok, W.; Elvitigala, K.C.M.L.; Nakahata, M.; Kojima, M.; Sakai, S. Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels. Cells 2022, 11, 881. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, Q.; Pinto, R.A.; Griebenow, K.; Schweitzer-Stenner, R.; Weber, W.J. Inactivation of horseradish peroxidase by phenoxyl radical attack. J. Am. Chem. Soc. 2005, 127, 1431–1437. [Google Scholar] [CrossRef]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Phenolic hydroxy groups incorporated for the peroxidase-catalyzed gelation of a carboxymethylcehulose support: Cellular adhesion and proliferation. Macromol. Biosci. 2009, 9, 262–267. [Google Scholar] [CrossRef]
- Carvalho, R.H.; Lemos, F.; Lemos, M.A.N.D.A.; Vojinović, V.; Fonseca, L.P.; Cabral, J.M.S. Kinetic modelling of phenol co-oxidation using horseradish peroxidase. Bioprocess Biosyst. Eng. 2006, 29, 99–108. [Google Scholar] [CrossRef]
- Reihmann, M.; Ritter, H. Synthesis of phenol polymers using peroxidases. In Enzyme-Catalyzed Synthesis of Polymers; Springer: Berlin/Heidelberg, Germany, 2006; Volume 194, pp. 1–49. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient degradation of high-molecular-weight hyaluronic acid by a combination of ultrasound, hydrogen peroxide, and copper ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Xu, A.; Xie, H.; Yu, W.; Xie, W.; Ma, X. Preparation of low molecular weight alginate by hydrogen peroxide depolymerization for tissue engineering. Carbohydr. Polym. 2010, 79, 660–664. [Google Scholar] [CrossRef]
- Takahashi, S.; Itoh, N.; Kawamura, Y.; Hayashi, R. Physical and Chemical Changes of Gelatins by Oxidation Treatment. Bull. Soc. Sci. Photogr. Jpn. 1998, 51, 22–28. (In Japanese) [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.C.; Cheng, F.H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- Burattini, S.; Ferri, R.; Battistelli, M.; Curci, R.; Luchetti, F.; Falcieri, E. C2C12 murine myoblasts as a model of skeletal muscle development: Morpho-functional characterization. Eur. J. Histochem. 2004, 48, 223–233. [Google Scholar]
- McMahon, D.K.; Anderson, P.A.W.; Bunting, J.B.; Saba, Z.; Oakeley, E.; Carolina, N.; Anderson, P.A.W.; Bunting, J.B.; Saba, Z.; Oakeley, A.E.; et al. C2C12 cells: Biophysical, biochemical and immunocytochemical properties. Am. J. Physiol.-Cell Physiol. 1994, 266, C1795–C1802. [Google Scholar] [CrossRef]
- Ikeda, K.; Ito, A.; Imada, R.; Sato, M.; Kawabe, Y.; Kamihira, M. In vitro drug testing based on contractile activity of C2C12 cells in an epigenetic drug model. Sci. Rep. 2017, 7, 44570. [Google Scholar] [CrossRef] [Green Version]
- Kondo, D.; Ogino, Y.; Ayukawa, Y.; Sakai, S.; Kawakami, K.; Koyano, K. Bone Regeneration of Tibial Defects in Rats with Enzymatic Hydrogelation of Gelatin Derivative and Recombinant Human Platelet-Derived Growth Factor-BB Complex. Int. J. Oral Maxillofac. Implant. 2013, 28, 1377–1385. [Google Scholar] [CrossRef] [Green Version]
- Le Thi, P.; Lee, Y.; Nguyen, D.H.; Park, K.D. In situ forming gelatin hydrogels by dual-enzymatic cross-linking for enhanced tissue adhesiveness. J. Mater. Chem. B 2017, 5, 757–764. [Google Scholar] [CrossRef]
- Agarwal, V.; Tjandra, E.S.; Iyer, K.S.; Humfrey, B.; Fear, M.; Wood, F.M.; Dunlop, S.; Raston, C.L. Evaluating the effects of nacre on human skin and scar cells in culture. Toxicol. Res. 2014, 3, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Catelas, I.; Sese, N.; Wu, B.M.; Dunn, J.C.Y.; Helgerson, S.; Tawil, B. Human mesenchymal stem cell proliferation and osteogenic differentiation in fibrin gels in vitro. Tissue Eng. 2006, 12, 2385–2396. [Google Scholar] [CrossRef]
- Wang, L.S.; Boulaire, J.; Chan, P.P.Y.; Chung, J.E.; Kurisawa, M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials 2010, 31, 8608–8616. [Google Scholar] [CrossRef]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [Green Version]
- Engler, A.; Bacakova, L.; Newman, C.; Hategan, A.; Griffin, M.; Discher, D. Substrate Compliance versus Ligand Density in Cell on Gel Responses. Biophys. J. 2004, 86, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Iskratsch, T.; Wolfenson, H.; Sheetz, M.P. Appreciating force and shape-the rise of mechanotransduction in cell biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 825–833. [Google Scholar] [CrossRef]
- Dupont, S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016, 343, 42–53. [Google Scholar] [CrossRef]
- Syed, S.; Karadaghy, A.; Zustiak, S. Simple polyacrylamide-based multiwell stiffness assay for the study of stiffness-dependent cell responses. J. Vis. Exp. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K. FAK-Dependent Cell Motility and Cell Elongation. Cells 2020, 9, 192. [Google Scholar] [CrossRef] [Green Version]
- Tomasch, J.; Maleiner, B.; Heher, P.; Rufin, M.; Andriotis, O.G.; Thurner, P.J.; Redl, H.; Fuchs, C.; Teuschl-Woller, A.H. Changes in Elastic Moduli of Fibrin Hydrogels Within the Myogenic Range Alter Behavior of Murine C2C12 and Human C25 Myoblasts Differently. Front. Bioeng. Biotechnol. 2022, 10, 836520. [Google Scholar] [CrossRef]
- Boonen, K.J.M.; Rosaria-Chak, K.Y.; Baaijens, F.P.T.; Van Der Schaft, D.W.J.; Post, M.J. Essential environmental cues from the satellite cell niche: Optimizing proliferation and differentiation. Am. J. Physiol.-Cell Physiol. 2009, 296, 1338–1345. [Google Scholar] [CrossRef] [Green Version]
- Romanazzo, S.; Forte, G.; Ebara, M.; Uto, K.; Pagliari, S.; Aoyagi, T.; Traversa, E.; Taniguchi, A. Substrate stiffness affects skeletal myoblast differentiation in vitro. Sci. Technol. Adv. Mater. 2012, 13, 064211. [Google Scholar] [CrossRef]
- Lacraz, G.; Rouleau, A.J.; Couture, V.; Söllrald, T.; Drouin, G.; Veillette, N.; Grandbois, M.; Grenier, G. Increased stiffness in aged skeletal muscle impairs muscle progenitor cell proliferative activity. PLoS ONE 2015, 10, e0136217. [Google Scholar] [CrossRef] [Green Version]
- Silver, J.S.; Günay, K.A.; Cutler, A.A.; Vogler, T.O.; Brown, T.E.; Pawlikowski, B.T.; Bednarski, O.J.; Bannister, K.L.; Rogowski, C.J.; McKay, A.G.; et al. Injury-mediated stiffening persistently activates muscle stem cells through YAP and TAZ mechanotransduction. Sci. Adv. 2021, 7, eabe4501. [Google Scholar] [CrossRef]
- Trensz, F.; Lucien, F.; Couture, V.; Söllrald, T.; Drouin, G.; Rouleau, A.J.; Grandbois, M.; Lacraz, G.; Grenier, G. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet. Muscle 2015, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Levy-Mishali, M.; Zoldan, J.; Levenberg, S. Effect of scaffold stiffness on myoblast differentiation. Tissue Eng.-Part A 2009, 15, 935–944. [Google Scholar] [CrossRef]
- Wang, P.Y.; Thissen, H.; Tsai, W.B. The roles of RGD and grooved topography in the adhesion, morphology, and differentiation of C2C12 skeletal myoblasts. Biotechnol. Bioeng. 2012, 109, 2104–2115. [Google Scholar] [CrossRef]
- Gribova, V.; Gauthier-Rouvière, C.; Albigès-Rizo, C.; Auzely-Velty, R.; Picart, C. Effect of RGD functionalization and stiffness modulation of polyelectrolyte multilayer films on muscle cell differentiation. Acta Biomater. 2013, 9, 6468–6480. [Google Scholar] [CrossRef] [Green Version]
- Robinson, P.A.; Brown, S.; McGrath, M.J.; Coghill, I.D.; Gurung, R.; Mitchell, C.A. Skeletal muscle LIM protein 1 regulates integrin-mediated myoblast adhesion, spreading, and migration. Am. J. Physiol.-Cell Physiol. 2003, 284, 681–695. [Google Scholar] [CrossRef] [Green Version]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef]
- Van Putten, S.; Shafieyan, Y.; Hinz, B. Mechanical control of cardiac myofibroblasts. J. Mol. Cell. Cardiol. 2016, 93, 133–142. [Google Scholar] [CrossRef]
- Hindi, S.M.; Tajrishi, M.M.; Kumar, A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013, 6, re2. [Google Scholar] [CrossRef] [Green Version]
- Nosenko, M.A.; Maluchenko, N.V.; Drutskaya, M.S.; Arkhipova, A.Y.; Agapov, I.I.; Nedospasov, S.A.; Moisenovich, M.M. Induction of ICAM-1 expression in mouse embryonic fibroblasts cultured on fibroin-gelatin scaffolds. Acta Nat. 2017, 9, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Pizza, F.X.; Martin, R.A.; Springer, E.M.; Leffler, M.S.; Woelmer, B.R.; Recker, I.J.; Leaman, D.W. Intercellular adhesion molecule-1 augments myoblast adhesion and fusion through homophilic trans-interactions. Sci. Rep. 2017, 7, 5094. [Google Scholar] [CrossRef] [Green Version]
- Goh, Q.; Dearth, C.L.; Corbett, J.T.; Pierre, P.; Chadee, D.N.; Pizza, F.X. Intercellular adhesion molecule-1 expression by skeletal muscle cells augments myogenesis. Exp. Cell Res. 2015, 331, 292–308. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, S.; Xu, Y.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; Song, W.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 5556–5568. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef]
- Hu, M.; Kurisawa, M.; Deng, R.; Teo, C.M.; Schumacher, A.; Thong, Y.X.; Wang, L.; Schumacher, K.M.; Ying, J.Y. Cell immobilization in gelatin-hydroxyphenylpropionic acid hydrogel fibers. Biomaterials 2009, 30, 3523–3531. [Google Scholar] [CrossRef]
- Wang, L.S.; Chung, J.E.; Pui-Yik Chan, P.; Kurisawa, M. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials 2010, 31, 1148–1157. [Google Scholar] [CrossRef]
- Peng, H.T.; Blostein, M.D.; Shek, P.N. Experimental optimization of an in situ forming hydrogel for hemorrhage control. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2009, 89, 199–209. [Google Scholar] [CrossRef]
- Asano, T.; Ishizua, T.; Yawo, H. Optically controlled contraction of photosensitive skeletal muscle cells. Biotechnol. Bioeng. 2012, 109, 199–204. [Google Scholar] [CrossRef]
- Asano, T.; Ishizuka, T.; Morishima, K.; Yawo, H. Optogenetic induction of contractile ability in immature C2C12 myotubes. Sci. Rep. 2015, 5, 8317. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarok, W.; Elvitigala, K.C.M.L.; Sakai, S. Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation. Gels 2022, 8, 387. https://doi.org/10.3390/gels8060387
Mubarok W, Elvitigala KCML, Sakai S. Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation. Gels. 2022; 8(6):387. https://doi.org/10.3390/gels8060387
Chicago/Turabian StyleMubarok, Wildan, Kelum Chamara Manoj Lakmal Elvitigala, and Shinji Sakai. 2022. "Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation" Gels 8, no. 6: 387. https://doi.org/10.3390/gels8060387
APA StyleMubarok, W., Elvitigala, K. C. M. L., & Sakai, S. (2022). Tuning Myogenesis by Controlling Gelatin Hydrogel Properties through Hydrogen Peroxide-Mediated Cross-Linking and Degradation. Gels, 8(6), 387. https://doi.org/10.3390/gels8060387