Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art
Abstract
:1. Introduction
2. Brief on PNIPAM-Based Hydrogel
3. Unique Properties of PNIPAM
4. Phase Transition for PNIPAMs
5. Preparation of PNIPAM-Based Hydrogel Systems
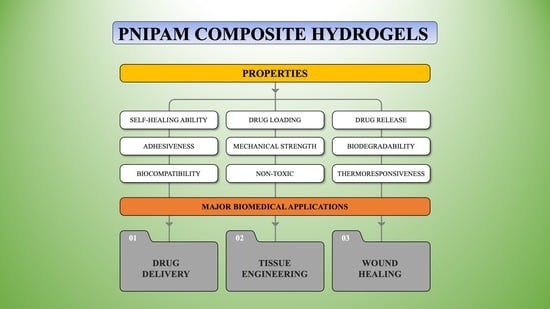
6. Formulation Approaches for Tailoring the Mechanical Behavior of PNIPAM Composite Hydrogel
7. PNIPAM-Based Hydrogels in Drug Delivery
8. Gene Delivery
9. PNIPAMs Hydrogel in Tissue Engineering
9.1. Cartilage Tissue Engineering
9.2. Bone Tissue Engineering
9.3. Cardiac Tissue Engineering
9.4. Lymphoid Tissue Engineering
9.5. Intestinal Tissue Engineering
10. PNIPAM Hydrogels for Wound Dressings
11. PNIPAM for Bioelectronics
12. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pramanik, S.; Sali, V. Connecting the dots in drug delivery: A tour d’horizon of chitosan-based nanocarriers system. Int. J. Biol. Macromol. 2021, 169, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, P.; Pramanik, S.; Vaidya, G.; Abdelgawad, M.A.; Ghoneim, M.M.; Singh, A.; Abualsoud, B.M.; Amaral, L.S.; Abourehab, M.A. Bacterial cellulose as a potential biopolymer in biomedical applications: A state-of-the-art review. J. Mater. Chem. B 2022, 10, 3199–3241. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.N.; Ferreira, L.M.B.; Cardoso, V.M.O.; Boni, F.I.; Souza, A.L.R.; Gremião, M.P.D. Recent advances in smart hydrogels for biomedical applications: From self-assembly to functional approaches. Eur. Polym. J. 2018, 99, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Darge, H.F.; Andrgie, A.T.; Tsai, H.C.; Lai, J.Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019, 133, 545–563. [Google Scholar] [CrossRef]
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773. [Google Scholar] [CrossRef]
- Di, X.; Kang, Y.; Li, F.; Yao, R.; Chen, Q.; Hang, C.; Xu, Y.; Wang, Y.; Sun, P.; Wu, G. Poly(N-isopropylacrylamide)/polydopamine/clay nanocomposite hydrogels with stretchability, conductivity, and dual light- and thermo- responsive bending and adhesive properties. Colloids Surf. B Biointerfaces 2019, 177, 149–159. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Abourehab, M.A.; Ansari, M.J.; Singh, A.; Hassan, A.; Abdelgawad, M.A.; Shrivastav, P.; Abualsoud, B.M.; Amaral, L.S.; Pramanik, S. Cubosomes as an emerging platform for drug delivery: A state-of-the-art review. J. Mater. Chem. B 2022, 10, 2781–2819. [Google Scholar] [CrossRef]
- Pramanik, S.; Mohanto, S.; Manne, R.; Rajendran, R.R.; Deepak, A.; Edapully, S.J.; Patil, T.; Katari, O. Nanoparticle-Based Drug Delivery System: The Magic Bullet for the Treatment of Chronic Pulmonary Diseases. Mol. Pharmaceutics 2021, 18, 3671–3718. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, Z.; Xian, C.; Yuan, Q.; Liu, G.; Wu, J. Natural Polymer-Based Hydrogels with Enhanced Mechanical Performances: Preparation, Structure, and Property. Adv. Healthc. Mater. 2019, 8, 1900670. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem.-A Eur. J. 2015, 21, 13164–13174. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kozlovskaya, V.; Medipelli, S.; Xue, B.; Ahmad, F.; Saeed, M.; Cropek, D.; Kharlampieva, E. Temperature-Sensitive Polymersomes for Controlled Delivery of Anticancer Drugs. Chem. Mater. 2015, 27, 7945–7956. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. pH/thermo-responsive poly(N-isopropylacrylamide-co-maleic acid) hydrogel with a sensor and an actuator for biomedical applications. Polymer 2017, 110, 177–186. [Google Scholar] [CrossRef]
- Karimi, M.; Ghasemi, A.; Zangabad, P.S.; Rahighi, R.; Basri, S.M.M.; Mirshekari, H.; Amiri, M.; Pishabad, Z.S.; Aslani, A.; Bozorgomid, M.; et al. Smart Micro/Nanoparticles in Stimulus-Responsive Drug/Gene Delivery Systems; Royal Society of Chemistry: London, UK, 2016; Volume 45. [Google Scholar] [CrossRef] [Green Version]
- Schmaljohann, D. Thermo- and pH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruel-Gariépy, E.; Leroux, J.-C. In situ-forming hydrogels—Review of temperature-sensitive systems. Eur. J. Pharm. Biopharm. Off. J. Arb. Fur Pharm. Verfahr. E.V. 2004, 58, 409–426. [Google Scholar] [CrossRef]
- Bajpai, A.K.; Shukla, S.K.; Bhanu, S.; Kankane, S. Responsive polymers in controlled drug delivery. Prog. Polym. Sci. 2008, 33, 1088–1118. [Google Scholar] [CrossRef]
- Karimi, M.; Sahandi Zangabad, P.; Ghasemi, A.; Amiri, M.; Bahrami, M.; Malekzad, H.; Ghahramanzadeh Asl, H.; Mahdieh, Z.; Bozorgomid, M.; Ghasemi, A.; et al. Temperature-responsive smart nanocarriers for delivery of therapeutic agents: Applications and recent advances. ACS Appl. Mater. Interfaces 2016, 8, 21107–21133. [Google Scholar] [CrossRef] [Green Version]
- Sang, Y.; Li, W.; Liu, H.; Zhang, L.; Wang, H.; Liu, Z.; Ren, J.; Qu, X. Construction of Nanozyme-Hydrogel for Enhanced Capture and Elimination of Bacteria. Adv. Funct. Mater. 2019, 29, 1900518. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.; Liu, S.; Wong, C.; Chu, S. Copolymer Brushes with Temperature-Triggered, Reversibly Switchable Bactericidal and Antifouling Properties for Biomaterial Surfaces. ACS Appl. Mater. Interfaces 2016, 8, 27207–27217. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, W.; Wang, R.; Yang, X.; Li, J.; Qiu, T.; Xiao, X. The preparation of green fluorescence-emissioned carbon dots/poly(N-isopropylacrylamide) temperature-sensitive hydrogels and research on their properties. Polymers 2019, 11, 1171. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.R.; Lee, S.L.; Park, S.N. Properties and in vitro drug release of pH-and temperature-sensitive double cross-linked interpenetrating polymer network hydrogels based on hyaluronic acid/poly (N-isopropylacrylamide) for transdermal delivery of luteolin. Int. J. Biol. Macromol. 2018, 118, 731–740. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.; Cha, C. Refined control of thermoresponsive swelling/deswelling and drug release properties of poly (N-isopropylacrylamide) hydrogels using hydrophilic polymer crosslinkers. J. Biomater. Sci. Polym. Ed. 2016, 27, 1698–1711. [Google Scholar] [CrossRef]
- Oak, M.; Mandke, R.; Singh, J. Smart polymers for peptide and protein parenteral sustained delivery. Drug Discov. Today Technol. 2012, 9, e131–e140. [Google Scholar] [CrossRef]
- Turturro, S.B.; Guthrie, M.J.; Appel, A.A.; Drapala, P.W.; Brey, E.M.; Pérez-Luna, V.H.; Mieler, W.F.; Kang-Mieler, J.J. The effects of cross-linked thermo-responsive PNIPAAm-based hydrogel injection on retinal function. Biomaterials 2011, 32, 3620–3626. [Google Scholar] [CrossRef]
- Gupta, M.K.; Martin, J.R.; Dollinger, B.R.; Hattaway, M.E.; Duvall, C.L. Thermogelling, ABC Triblock Copolymer Platform for Resorbable Hydrogels with Tunable, Degradation-Mediated Drug Release. Adv. Funct. Mater. 2017, 27, 1–14. [Google Scholar] [CrossRef]
- McCune, J.A.; Mommer, S.; Parkins, C.C.; Scherman, O.A. Design Principles for Aqueous Interactive Materials: Lessons from Small Molecules and Stimuli-Responsive Systems. Adv. Mater. 2020, 32, 1–14. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, C.; Yang, X.; Shen, B.; Sun, Y.; Chen, Y.; Xu, X.; Sun, H.; Yu, K.; Yang, B.; et al. PH- and Temperature-Sensitive Hydrogel Nanoparticles with Dual Photoluminescence for Bioprobes. ACS Nano 2016, 10, 5856–5863. [Google Scholar] [CrossRef]
- Ziane, S.; Schlaubitz, S.; Miraux, S.; Patwa, A.; Lalande, C.; Bilem, I.; Lepreux, S.; Rousseau, B.; Le Meins, J.F.; Latxague, L. A thermosensitive low molecular weight hydrogel as scaffold for tissue engineering. Eur. Cells Mater. 2012, 23, 147–160. [Google Scholar] [CrossRef]
- Gan, J.; Guan, X.; Zheng, J.; Guo, H.; Wu, K.; Liang, L.; Lu, M. Biodegradable, thermoresponsive PNIPAM-based hydrogel scaffolds for the sustained release of levofloxacin. RSC Adv. 2016, 6, 32967–32978. [Google Scholar] [CrossRef]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Y.; Fu, W.; Yao, M.; Ding, Z.; Xuan, J.; Li, D.; Wang, S.; Xia, Y.; Cao, M. Poly (N-isopropylacrylamide)-based thermoresponsive composite hydrogels for biomedical applications. Polymers 2020, 12, 580. [Google Scholar] [CrossRef] [Green Version]
- Tokarev, I.; Minko, S. Stimuli-responsive hydrogel thin films. Soft. Matter. 2009, 5, 511–524. [Google Scholar] [CrossRef]
- Alexander, A.; Khan, J.; Saraf, S.; Saraf, S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2014, 88, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Mohanty, M.; Umashankar, P.R.; Jayakrishnan, A. Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatin. Biomaterials 2005, 26, 6335–6342. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Ghobril, C.; Grinstaff, M.W. The chemistry and engineering of polymeric hydrogel adhesives for wound closure: A tutorial. Chem. Soc. Rev. 2015, 44, 1820–1835. [Google Scholar] [CrossRef]
- Ghavaminejad, A.; Park, C.H.; Kim, C.S. In Situ Synthesis of Antimicrobial Silver Nanoparticles within Antifouling Zwitterionic Hydrogels by Catecholic Redox Chemistry for Wound Healing Application. Biomacromolecules 2016, 17, 1213–1223. [Google Scholar] [CrossRef]
- Singer, A.J.; Dagum, A.B. Current Management of Acute Cutaneous Wounds. N. Engl. J. Med. 2008, 359, 1037–1046. [Google Scholar] [CrossRef]
- Chang, Y.; Yandi, W.; Chen, W.Y.; Shih, Y.J.; Yang, C.C.; Chang, Y.; Ling, Q.D.; Higuchi, A. Tunable bioadhesive copolymer hydrogels of thermoresponsive poly(N-isopropyl acrylamide) containing zwitterionic polysulfobetaine. Biomacromolecules 2010, 11, 1101–1110. [Google Scholar] [CrossRef]
- Mi, L.; Xue, H.; Li, Y.; Jiang, S. A thermoresponsive antimicrobial wound dressing hydrogel based on a cationic betaine ester. Adv. Funct. Mater. 2011, 21, 4028–4034. [Google Scholar] [CrossRef]
- Vihola, H.; Laukkanen, A.; Valtola, L.; Tenhu, H.; Hirvonen, J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam); amphiphilically modified poly(N-vinylcaprolactam). Biomaterials 2005, 26, 3055–3064. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Cunliffe, D.; Alarcón, C.d.; Peters, V.; Smith, J.R.; Alexander, C. Thermoresponsive surface-grafted poly(N-isopropylacrylamide) copolymers: Effect of phase transitions on protein and bacterial attachment. Langmuir 2003, 19, 2888–2899. [Google Scholar] [CrossRef]
- Nie, L.; Li, J.; Lu, G.; Wei, X.; Deng, Y.; Liu, S.; Zhong, S.; Shi, Q.; Hou, R.; Sun, Y.; et al. Temperature responsive hydrogel for cells encapsulation based on graphene oxide reinforced poly(N-isopropylacrylamide)/hydroxyethyl-chitosan. Mater. Today Commun. 2022, 31, 103697. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.L.; Cao, F.H.; Wang, J.L.; Yu, Z.L.; Ge, J.; Lu, Y.; Wang, Z.H.; Yu, S.H. Highly Stimuli-Responsive Au Nanorods/Poly(N-isopropylacrylamide) (PNIPAM) Composite Hydrogel for Smart Switch. ACS Appl. Mater. Interfaces 2017, 9, 24857–24863. [Google Scholar] [CrossRef]
- Wang, C.; Flynn, N.T.; Langer, R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv. Mater. 2004, 16, 1074–1079. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef]
- Singh, N.K.; Lee, D.S. In situ gelling pH- and temperature-sensitive biodegradable block copolymer hydrogels for drug delivery. J. Control. Release 2014, 193, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Liu, X.; Miller, A.L.; Cheng, Y.S.; Yeh, M.L.; Lu, L. Strengthening injectable thermo-sensitive NIPAAm-g-chitosan hydrogels using chemical cross-linking of disulfide bonds as scaffolds for tissue engineering. Carbohydr. Polym. 2018, 192, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ramirez, C.M.; Miljkovic, N.; Li, H.; Rubin, J.P.; Marra, K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials 2009, 30, 6844–6853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadoo, N.; Gramlich, W.M. Spatiotemporal Modification of Stimuli-Responsive Hyaluronic Acid/Poly(N-isopropylacrylamide) Hydrogels. ACS Biomater. Sci. Eng. 2016, 2, 1341–1350. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Q.; Du, C.; Peng, R.; Hua, Y.; Li, Q.; Hu, A.; Zhou, J. Preparation and Anti-Mold Properties of Nano-ZnO/Poly(N-isopropylacrylamide) Composite Hydrogels. Molecules 2020, 25, 4135. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Sharma, L. In Situ Formation of Silver Nanoparticles in Poly(N-isopropyl Acrylamide) Hydrogel for Antibacterial Applications. Des. Monomers Polym. 2011, 14, 383–394. [Google Scholar] [CrossRef]
- Wei, J.; He, P.; Liu, A.; Chen, X.; Wang, X.; Jing, X. Surface Modification of Hydroxyapatite Nanoparticles with Thermal-Responsive PNIPAM by ATRP. Macromol. Biosci. 2009, 9, 1237–1246. [Google Scholar] [CrossRef]
- Liu, X.; Song, T.; Chang, M.; Meng, L.; Wang, X.; Sun, R.; Ren, J. Carbon Nanotubes Reinforced Maleic Anhydride-Modified Xylan-g-Poly(N-isopropylacrylamide) Hydrogel with Multifunctional Properties. Materials 2018, 11, 354. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Xu, H.; Che, L.; Sha, D.; Huang, C.; Meng, T.; Song, D. Application of Inorganic Nanocomposite Hydrogels in Bone Tissue Engineering. iScience 2020, 23, 101845. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.-J.; Jia, S.-R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Matricardi, P.; di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating polymer networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Kolahchi, A.R.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Sosnik, A.; Seremeta, K.P. Polymeric hydrogels as technology platform for drug delivery applications. Gels 2017, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Hoffman, A.S. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Control. Release 1987, 6, 297–305. [Google Scholar] [CrossRef]
- Tang, L.; Wang, L.; Yang, X.; Feng, Y.; Li, Y.; Feng, W. Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Prog. Mater. Sci. 2021, 115, 100702. [Google Scholar] [CrossRef]
- Ju, G.; Cheng, M.; Xiao, M.; Xu, J.; Pan, K.; Wang, X.; Zhang, Y.; Shi, F. Smart Transportation Between Three Phases Through a Stimulus-Responsive Functionally Cooperating Device. Adv. Mater. 2013, 25, 2915–2919. [Google Scholar] [CrossRef]
- Sun, T.; Song, W.; Jiang, L. Control over the responsive wettability of poly(N-isopropylacrylamide) film in a large extent by introducing an irresponsive molecule. Chem. Commun. 2005, 13, 1723–1725. [Google Scholar] [CrossRef]
- Füllbrandt, M.; Ermilova, E.; Asadujjaman, A.; Hölzel, R.; Bier, F.F.; von Klitzing, R.; Schönhals, A. Dynamics of Linear Poly(N-isopropylacrylamide) in Water around the Phase Transition Investigated by Dielectric Relaxation Spectroscopy. J. Phys. Chem. B 2014, 118, 3750–3759. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Cao, C.; Chen, K.; Wen, Y.; Fang, D.; Li, L.; Guo, X. Binary Solvent Colloids of Thermosensitive Poly(N-isopropylacrylamide) Microgel for Smart Windows. Ind. Eng. Chem. Res. 2014, 53, 18462–18472. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yang, Y.Y.; Chung, T.S. The influence of cold treatment on properties of temperature-sensitive poly(N-isopropylacrylamide) hydrogels. J. Colloid Interface Sci. 2002, 246, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, E.S.; Tanaka, T. Kinetics of discontinuous volume-phase transition of gels. J. Chem. Phys. 1988, 89, 1695–1703. [Google Scholar] [CrossRef]
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Xu, X.D.; Cheng, S.X.; Zhuo, R.X. Strategies to improve the response rate of thermosensitive PNIPAAm hydrogels. Soft. Matter. 2008, 4, 385–391. [Google Scholar] [CrossRef]
- Sayil, C.; Okay, O. Macroporous poly (N-isopropylacrylamide) networks. Polym. Bull. 2002, 506, 499–506. [Google Scholar] [CrossRef]
- Zhang, J.T.; Cheng, S.X.; Huang, S.W.; Zhuo, R.X. Temperature-sensitive poly (N-isopropylacrylamide) hydrogels with macroporous structure and fast response rate. Macromol. Rapid Commun. 2003, 24, 447–451. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Wang, F.J.; Chu, C.C. Thermoresponsive hydrogel with rapid response dynamics. J. Mater. Sci. Mater. Med. 2003, 14, 451–455. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Wu, D.Q.; Chu, C.C. Synthesis, characterization and controlled drug release of thermosensitive IPN-PNIPAAm hydrogels. Biomaterials 2004, 25, 3793–3805. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Yang, Y.Y.; Wang, F.J.; Chung, T.S. Thermosensitive poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with expanded network structures and improved oscillating swelling-deswelling properties. Langmuir 2002, 18, 2013–2018. [Google Scholar] [CrossRef]
- Ju, H.K.; Kim, S.Y.; Lee, Y.M. pH/temperature-responsive behaviors of semi-IPN and comb-type graft hydrogels composed of alginate and poly(N-isopropylacrylamide). Polymer 2001, 42, 6851–6857. [Google Scholar] [CrossRef]
- Vázquez-Dorbatt, V.; Tolstyka, Z.P.; Maynard, H.D. Synthesis of aminooxy end-functionalized pnipaam by raft polymerization for protein and polysaccharide conjugation. Macromolecules 2009, 42, 7650–7656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.T.; Cheng, S.X.; Zhuo, R.X. Poly(vinyl alcohol)/poly(N-isopropylacrylamide) semi-interpenetrating polymer network hydrogels with rapid response to temperature changes. Colloid Polym. Sci. 2003, 281, 580–583. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Ma, H.; Lu, X.; Shi, M.; Li, N.; Ye, M. Preparation and characterization of pH- and temperature-responsive nanocomposite double network hydrogels. Mater. Sci. Eng. C 2013, 33, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Bin Imran, A.; Esaki, K.; Gotoh, H.; Seki, T.; Ito, K.; Sakai, Y.; Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, Y.; Wang, W.; Ji, Q.; Xia, Y. Temperature-sensitive poly(N-isopropylacrylamide)/graphene oxide nanocomposite hydrogels by in situ polymerization with improved swelling capability and mechanical behavior. Eur. Polym. J. 2013, 49, 389–396. [Google Scholar] [CrossRef]
- Lencina, M.S.; Iatridi, Z.; Villar, M.A.; Tsitsilianis, C. Thermoresponsive hydrogels from alginate-based graft copolymers. Eur. Polym. J. 2014, 61, 33–44. [Google Scholar] [CrossRef]
- Takigawa, T.; Yamawaki, T.; Takahashi, K.; Masuda, T. Change in Young’s modulus of poly(N-isopropylacrylamide) gels by volume phase transition. Polym. Gels Netw. 1998, 5, 585–589. [Google Scholar] [CrossRef]
- Rivero, R.E.; Capella, V.; Liaudat, A.C.; Bosch, P.; Barbero, C.A.; Rodríguez, N.; Rivarola, C.R. Mechanical and physicochemical behavior of a 3D hydrogel scaffold during cell growth and proliferation. RSC Adv. 2020, 10, 5827–5837. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Zhuo, R.X.; Cui, J.Z.; Zhang, J.T. A novel thermo-responsive drug delivery system with positive controlled release. Int. J. Pharm. 2002, 235, 43–50. [Google Scholar] [CrossRef]
- Gutowska, A.; Bark, J.S.; Kwon, I.C.; Bae, Y.H.; Cha, Y.; Kim, S.W. Squeezing hydrogels for controlled oral drug delivery. J. Control. Release 1997, 48, 141–148. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Concheiro, A. Reversible adsorption by a pH- and temperature-sensitive acrylic hydrogel. J. Control. Release 2002, 80, 247–257. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Boere, K.W.M.; Soliman, B.G.; Rijkers, D.T.S.; Hennink, W.E.; Vermonden, T. Thermoresponsive injectable hydrogels cross-linked by native chemical ligation. Macromolecules 2014, 47, 2430–2438. [Google Scholar] [CrossRef]
- Gao, C.; Ren, J.; Zhao, C.; Kong, W.; Dai, Q.; Chen, Q.; Liu, C.; Sun, R. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef]
- Charan, H.; Kinzel, J.; Glebe, U.; Anand, D.; Garakani, T.M.; Zhu, L.; Bocola, M.; Schwaneberg, U.; Böker, A. Grafting PNIPAAm from β-barrel shaped transmembrane nanopores. Biomaterials 2016, 107, 115–123. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Matsushita, S.; Guan, J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials 2011, 32, 3220–3232. [Google Scholar] [CrossRef]
- Yang, J.; van Lith, R.; Baler, K.; Hoshi, R.A.; Ameer, G.A. A thermoresponsive biodegradable polymer with intrinsic antioxidant properties. Biomacromolecules 2014, 15, 3942–3952. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.; Ping, Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control. Release 2007, 120, 186–194. [Google Scholar] [CrossRef]
- Das, D.; Ghosh, P.; Ghosh, A.; Haldar, C.; Dhara, S.; Panda, A.B.; Pal, S. Stimulus-Responsive, Biodegradable, Biocompatible, Covalently Cross-Linked Hydrogel Based on Dextrin and Poly(N -isopropylacrylamide) for in Vitro/in Vivo Controlled Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 14338–14351. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Shikata, T. Hydration and dynamic behavior of poly (N-isopropylacrylamide) s in aqueous solution: A sharp phase transition at the lower critical solution temperature. J. Am. Chem. Soc. 2006, 128, 10030–10031. [Google Scholar] [CrossRef] [PubMed]
- Yanase, K.; Buchner, R.; Sato, T. Microscopic insights into the phase transition of poly (N-isopropylacrylamide) in aqueous media: Effects of molecular weight and polymer concentration. J. Mol. Liq. 2020, 302, 112025. [Google Scholar] [CrossRef]
- P.R. ten Wolde; Chandler, D. Drying-induced hydrophobic polymer collapse. Proc. Natl. Acad. Sci. USA 2002, 99, 6539–6543. [Google Scholar] [CrossRef] [Green Version]
- Futscher, M.H.; Philipp, M.; Müller-Buschbaum, P.; Schulte, A. The Role of Backbone Hydration of Poly(N-isopropyl acrylamide) Across the Volume Phase Transition Compared to its Monomer. Sci. Rep. 2017, 7, 17012. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Wu, P. LCST transition of PNIPAM-b-PVCL in water: Cooperative aggregation of two distinct thermally responsive segments. Soft. Matter. 2014, 10, 3578–3586. [Google Scholar] [CrossRef]
- Kojima, H.; Tanaka, F. Cooperative Hydration Induces Discontinuous Volume Phase Transition of Cross-Linked Poly(N-isopropylacrylamide) Gels in Water. Macromolecules 2010, 43, 5103–5113. [Google Scholar] [CrossRef]
- Grinberg, V.Y.; Burova, T.V.; Grinberg, N.V.; Moskalets, A.P.; Dubovik, A.S.; Plashchina, I.G.; Khokhlov, A.R. Energetics and Mechanisms of poly(N-isopropylacrylamide) Phase Transitions in Water–Methanol Solutions. Macromolecules 2020, 53, 10765–10772. [Google Scholar] [CrossRef]
- Shan, J.; Chen, J.; Nuopponen, M.; Tenhu, H. Two phase transitions of poly(N-isopropylacrylamide) brushes bound to gold nanoparticles. Langmuir ACS J. Surf. Colloids 2004, 20, 4671–4676. [Google Scholar] [CrossRef]
- Rey, M.; Hou, X.; Tang, J.S.J.; Vogel, N. Interfacial arrangement and phase transitions of PNiPAm microgels with different crosslinking densities. Soft. Matter. 2017, 13, 8717–8727. [Google Scholar] [CrossRef]
- Okada, Y.; Tanaka, F.; Kujawa, P.; Winnik, F.M. Unified model of association-induced lower critical solution temperature phase separation and its application to solutions of telechelic poly(ethylene oxide) and of telechelic poly(N-isopropylacrylamide) in water. J. Chem. Phys. 2006, 125, 244902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, Q.-T.; Yao, Z.-H.; Chang, Y.-T.; Wang, F.-M.; Chern, C.-S. LCST phase transition kinetics of aqueous poly(N-isopropylacrylamide) solution. J. Taiwan Inst. Chem. Eng. 2018, 93, 63–69. [Google Scholar] [CrossRef]
- Bischofberger, I.; Trappe, V. New aspects in the phase behaviour of poly-N-isopropyl acrylamide: Systematic temperature dependent shrinking of PNiPAM assemblies well beyond the LCST. Sci. Rep. 2015, 5, 15520. [Google Scholar] [CrossRef] [PubMed]
- Werner, P.; Münzberg, M.; Hass, R.; Reich, O. Process analytical approaches for the coil-to-globule transition of poly(N-isopropylacrylamide) in a concentrated aqueous suspension. Anal. Bioanal. Chem. 2017, 409, 807–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Tong, Z.; Lyon, L.A. Control of poly(N-isopropylacrylamide) microgel network structure by precipitation polymerization near the lower critical solution temperature. Langmuir ACS J. Surf. Colloids 2011, 27, 4142–4148. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.; Park, H.-K.; Park, H.; Lee, S.-H. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: Role in drug delivery and tissue engineering. Macromol. Res. 2016, 24, 297–304. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Liang, J.; Zhang, K.; Li, J. Hydrogel Preparation Methods and Biomaterials for Wound Dressing. Life 2021, 11, 1016. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Ha, S.; Lee, J.; Premkumar, T.; Song, C. UV-mediated synthesis of pNIPAM-crosslinked double-network alginate hydrogels: Enhanced mechanical and shape-memory properties by metal ions and temperature. Polymer 2018, 149, 206–212. [Google Scholar] [CrossRef]
- Zarzyka, I.; Pyda, M.; di Lorenzo, M.L. Influence of crosslinker and ionic comonomer concentration on glass transition and demixing/mixing transition of copolymers poly(N-isopropylacrylamide) and poly(sodium acrylate) hydrogels. Colloid Polym. Sci. 2014, 292, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.M.; Li, R.; Ren, J.; Lv, X.C.; Zhao, X.H.; Ji, Q.; Xia, Y.Z. Restorable high-strength poly(N-isopropylacrylamide) hydrogels constructed through chitosan-based dual macro-cross-linkers with rapid response to temperature jumps. RSC Adv. 2017, 7, 47767–47774. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, C.; Zhang, W.; Yin, Y.; Rao, Q. Preparation and characterization of PAM/SA tough hydrogels reinforced by IPN technique based on covalent/ionic crosslinking. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Bi, S.; Pang, J.; Huang, L.; Sun, M.; Cheng, X.; Chen, X. The toughness chitosan-PVA double network hydrogel based on alkali solution system and hydrogen bonding for tissue engineering applications. Int. J. Biol. Macromol. 2020, 146, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, M.; Zhang, L.; He, B.; Chen, X.; Sun, J. Facile synthesis of self-healing and layered sodium alginate/polyacrylamide hydrogel promoted by dynamic hydrogen bond. Carbohydr. Polym. 2021, 256, 117580. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xia, Y.; Zhang, D.; Sun, X.; Chen, X.; Oliver, S.; Shi, S.; Lei, L. Hydrogen-Bonding Reinforced Injectable Hydrogels: Application as a Thermo-Triggered Drug Controlled-Release System. ACS Appl. Polym. Mater. 2020, 2, 1587–1596. [Google Scholar] [CrossRef]
- Xiao, J.; Zhou, Y.; Ye, M.; An, Y.; Wang, K.; Wu, Q.; Song, L.; Zhang, J.; He, H.; Zhang, Q.; et al. Freeze-Thawing Chitosan/Ions Hydrogel Coated Gauzes Releasing Multiple Metal Ions on Demand for Improved Infected Wound Healing. Adv. Healthc. Mater. 2021, 10, e2001591. [Google Scholar] [CrossRef]
- Oh, S.H.; An, D.B.; Kim, T.H.; Lee, J.H. Wide-range stiffness gradient PVA/HA hydrogel to investigate stem cell differentiation behavior. Acta Biomater. 2016, 35, 23–31. [Google Scholar] [CrossRef]
- Genevro, G.M.; de Moraes, M.A.; Beppu, M.M. Freezing influence on physical properties of glucomannan hydrogels. Int. J. Biol. Macromol. 2019, 128, 401–405. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Peñas, F.J.; Zavala-Rivera, P.; Rosas-Durazo, A.J.; Maldonado-Arce, A.D.; Martínez-Barbosa, M.E. Effect of freeze-thawing conditions for preparation of chitosan-poly (vinyl alcohol) hydrogels and drug release studies. Carbohydr. Polym. 2018, 195, 476–485. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Tao, Y.; Deng, C.; Yu, K.; Zhang, W.; Deng, L.; Xiong, W. Two-Step Freezing Polymerization Method for Efficient Synthesis of High-Performance Stimuli-Responsive Hydrogels. ACS Omega 2020, 5, 5921–5930. [Google Scholar] [CrossRef] [Green Version]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheaburu-Yilmaz, C.N.; Yilmaz, O.; Kose, F.A.; Bibire, N. Chitosan-Graft-Poly(N-isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers 2019, 11, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Q.; Duan, L.; Zou, M.; Chen, X.; Gao, G.H. The tough allograft adhesive behavior between polyacrylamide and poly(acrylic acid) hydrophobic association hydrogels. Mater. Chem. Phys. 2017, 193, 57–62. [Google Scholar] [CrossRef]
- Cho, E.C.; Lee, J.; Cho, K. Role of Bound Water and Hydrophobic Interaction in Phase Transition of Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules 2003, 36, 9929–9934. [Google Scholar] [CrossRef]
- Custodio, K.K.S.; Claudio, G.C.; Nellas, R.B. Structural Dynamics of Neighboring Water Molecules of N-isopropylacrylamide Pentamer. ACS Omega 2020, 5, 1408–1413. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Lim, S.I. Bioinspired tunable hydrogels: An update on methods of preparation, classification, and biomedical and therapeutic applications. Int. J. Pharm. 2022, 612, 121368. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Kim, H.; Yoon, J. Photothermally driven fast responding photo-actuators fabricated with comb-type hydrogels and magnetite nanoparticles. Sci. Rep. 2015, 5, 15124. [Google Scholar] [CrossRef] [Green Version]
- Rana, M.M.; Rajeev, A.; Natale, G.; Siegler, H.D. Effects of synthesis-solvent polarity on the physicochemical and rheological properties of poly(N-isopropylacrylamide) (PNIPAm) hydrogels. J. Mater. Res. Technol. 2021, 13, 769–786. [Google Scholar] [CrossRef]
- Li, Q.-F.; Du, X.; Jin, L.; Hou, M.; Wang, Z.; Hao, J. Highly luminescent hydrogels synthesized by covalent grafting of lanthanide complexes onto PNIPAM via one-pot free radical polymerization. J. Mater. Chem. C 2016, 4, 3195–3201. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, X.; Cong, Y.; Liu, L.; Li, L. Free Radical Polymerization of Gold Nanoclusters and Hydrogels for Cell Capture and Light-Controlled Release. ACS Appl. Mater. Interfaces 2021, 13, 19360–19368. [Google Scholar] [CrossRef]
- Kim, S.; Healy, K.E. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide-co-acrylic acid) Hydrogels with Proteolytically Degradable Cross-Links. Biomacromolecules 2003, 4, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Schachschal, S.; Adler, H.-J.; Pich, A.; Wetzel, S.; Matura, A.; van Pee, K.-H. Encapsulation of enzymes in microgels by polymerization/cross-linking in aqueous droplets. Colloid Polym. Sci. 2011, 289, 693–698. [Google Scholar] [CrossRef]
- Yi, G.; Huang, Y.; Xiong, F.; Liao, B.; Yang, J.; Chen, X. Preparation and swelling behaviors of rapid responsive semi-IPN NaCMC/PNIPAm hydrogels. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2011, 26, 1073–1078. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Wiwatsamphan, P.; Chirachanchai, S. Persistently reversible pH-/thermo-responsive chitosan/poly (N-isopropyl acrylamide) hydrogel through clickable crosslinked interpenetrating network. Polym. Degrad. Stab. 2022, 198, 109874. [Google Scholar] [CrossRef]
- Gwon, S.; Park, S. Preparation of uniformly sized interpenetrating polymer network polyelectrolyte hydrogel droplets from a solid-state liquid crystal shell. J. Ind. Eng. Chem. 2021, 99, 235–245. [Google Scholar] [CrossRef]
- Menegatti, T.; Žnidaršič-Plazl, P. Copolymeric Hydrogel-Based Immobilization of Yeast Cells for Continuous Biotransformation of Fumaric Acid in a Microreactor. Micromachines 2019, 10, 867. [Google Scholar] [CrossRef] [Green Version]
- Kondiah, P.J.; Choonara, Y.E.; Kondiah, P.P.; Marimuthu, T.; Kumar, P.; Du Toit, L.C.; Pillay, V. A Review of Injectable Polymeric Hydrogel Systems for Application in Bone Tissue Engineering. Molecules 2016, 21, 1580. [Google Scholar] [CrossRef] [Green Version]
- Vinchhi, P.; Rawal, S.U.; Patel, M.M. Chapter 19—Biodegradable hydrogels. In Drug Delivery Devices and Therapeutic Systems; Chappel, E., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 395–419. [Google Scholar] [CrossRef]
- Boyes, V.L.; Janani, R.; Partridge, S.; Fielding, L.A.; Breen, C.; Foulkes, J.; Le Maitre, C.L.; Sammon, C. One-pot precipitation polymerisation strategy for tuneable injectable Laponite®-pNIPAM hydrogels: Polymerisation, processability and beyond. Polymer 2021, 233, 124201. [Google Scholar] [CrossRef]
- Ting, M.S.; Vella, J.; Raos, B.J.; Narasimhan, B.N.; Svirskis, D.; Travas-Sejdic, J.; Malmström, J. Conducting polymer hydrogels with electrically-tuneable mechanical properties as dynamic cell culture substrates. Biomater. Adv. 2022, 134, 112559. [Google Scholar] [CrossRef]
- Xiao, Q.; Cui, Y.; Meng, Y.; Guo, F.; Ruan, X.; He, G.; Jiang, X. PNIPAm hydrogel composite membrane for high-throughput adsorption of biological macromolecules. Sep. Purif. Technol. 2022, 294, 121224. [Google Scholar] [CrossRef]
- Liu, J.; Miao, J.; Zhao, L.; Liu, Z.; Leng, K.; Xie, W.; Yu, Y. Versatile Bilayer Hydrogel for Wound Dressing through PET-RAFT Polymerization. Biomacromolecules 2022, 23, 1112–1123. [Google Scholar] [CrossRef]
- Rafieian, S.; Mirzadeh, H.; Mahdavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Chakraborty, I.; Bodurtha, K.J.; Heeder, N.J.; Godfrin, M.P.; Tripathi, A.; Hurt, R.H.; Shukla, A.; Bose, A. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhao, Q.; Wang, Y.; Zhu, S.; Wang, Z. Cellulose nanocrystals reinforced highly stretchable thermal-sensitive hydrogel with ultra-high drug loading. Carbohydr. Polym. 2021, 266, 118122. [Google Scholar] [CrossRef]
- Lin, X.; Guan, X.; Wu, Y.; Zhuang, S.; Wu, Y.; Du, L.; Zhao, J.; Rong, J.; Zhao, J.; Tu, M. An alginate/poly(N-isopropylacrylamide)-based composite hydrogel dressing with stepwise delivery of drug and growth factor for wound repair. Mater. Sci. Engineering. C Mater. Biol. Appl. 2020, 115, 111123. [Google Scholar] [CrossRef]
- Han, L.; Zhang, Y.; Lu, X.; Wang, K.; Wang, Z.; Zhang, H. Polydopamine nanoparticles modulating stimuli-responsive PNIPAM hydrogels with cell/tissue adhesiveness. ACS Appl. Mater. Interfaces 2016, 8, 29088–29100. [Google Scholar] [CrossRef]
- Geng, S.; Zhao, H.; Zhan, G.; Zhao, Y.; Yang, X. Injectable in situ forming hydrogels of thermosensitive polypyrrole nanoplatforms for precisely synergistic photothermo-chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 7995–8005. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly (N-isopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech 2019, 20, 119. [Google Scholar] [CrossRef]
- Spicer, C.D. Hydrogel scaffolds for tissue engineering: The importance of polymer choice. Polym. Chem. 2020, 11, 184–219. [Google Scholar] [CrossRef]
- McInnes, S.J.; Szili, E.J.; Al-Bataineh, S.A.; Vasani, R.B.; Xu, J.; Alf, M.E.; Gleason, K.K.; Short, R.D.; Voelcker, N.H. Fabrication and Characterization of a Porous Silicon Drug Delivery System with an Initiated Chemical Vapor Deposition Temperature-Responsive Coating. Langmuir 2016, 32, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Lu, L.; Wang, Q.; Benicewicz, B.C. PH and Thermal Dual-Responsive Nanoparticles for Controlled Drug Delivery with High Loading Content. ACS Omega 2017, 2, 3399–3405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta, M.E.; Jadhav, S.A.; Magnacca, G.; Scalarone, D.; Mártire, D.O.; Parolo, M.E.; Carlos, L. Synthesis and in vitro testing of thermoresponsive polymer-grafted core-shell magnetic mesoporous silica nanoparticles for efficient controlled and targeted drug delivery. J. Colloid Interface Sci. 2019, 544, 198–205. [Google Scholar] [CrossRef]
- Li, P.; Hou, X.; Qu, L.; Dai, X.; Zhang, C. PNIPAM-MAPOSS hybrid hydrogels with excellent swelling behavior and enhanced mechanical performance: Preparation and drug release of 5-fluorouracil. Polymers 2018, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, L.; Zhang, H.; Yang, Y.; Liu, X.; Chen, Y. Temperature and magnetism bi-responsive molecularly imprinted polymers: Preparation, adsorption mechanism and properties as drug delivery system for sustained release of 5-fluorouracil. Mater. Sci. Eng. C 2016, 61, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.D.; Guerrero, S.; Benito, M.; Fernández, A.; Teijón, C.; Olmo, R.; Katime, I.; Teijón, J.M. In vitro and in vivo evaluation of a folate-targeted copolymeric submicrohydrogel based on N-isopropylacrylamide as 5-fluorouracil delivery system. Polymers 2011, 3, 1107–1125. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Pinel, B.; Ortega-Rodríguez, A.; Porras-Alcalá, C.; Cabeza, L.; Contreras-Cáceres, R.; Ortiz, R.; Díaz, A.; Moscoso, A.; Sarabia, F.; Prados, J.; et al. Magnetically active pNIPAM nanosystems as temperature-sensitive biocompatible structures for controlled drug delivery. Artif. Cells Nanomed. Biotechnol. 2020, 48, 1022–1035. [Google Scholar] [CrossRef]
- Wang, N.; Shi, J.; Wu, C.; Chu, W.; Tao, W.; Li, W.; Yuan, X. Design of DOX-GNRs-PNIPAM@PEG-PLA Micelle With Temperature and Light Dual-Function for Potent Melanoma Therapy. Front. Chem. 2021, 8, 1181. [Google Scholar] [CrossRef]
- Rezaei, F.; Damoogh, S.; Reis, R.L.; Kundu, S.C.; Mottaghitalab, F.; Farokhi, M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 2020, 13, 15005. [Google Scholar] [CrossRef]
- Erkisa, M.; Ari, F.; Ulku, I.; Khodadust, R.; Yar, Y.; Yagci Acar, H.; Ulukaya, E. Etoposide Loaded SPION-PNIPAM Nanoparticles Improve the in vitro Therapeutic Outcome on Metastatic Prostate Cancer Cells via Enhanced Apoptosis. Chem. Biodivers. 1974, 17, 109867. [Google Scholar] [CrossRef] [PubMed]
- Safakas, K.; Saravanou, S.-F.; Iatridi, Z.; Tsitsilianis, C. Alginate-g-PNIPAM-Based Thermo/Shear-Responsive Injectable Hydrogels: Tailoring the Rheological Properties by Adjusting the LCST of the Grafting Chains. Int. J. Mol. Sci. 2021, 22, 3824. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Téllez, C.N.; Luque-Alcaraz, A.G.; Plascencia-Jatomea, M.; Higuera-Valenzuela, H.J.; Burgos-Hernández, M.; García-Flores, N.; Álvarez-Ramos, M.E.; Iriqui-Razcon, J.L.; Gonzalez, R.E.; Hernández-Abril, P.A. Synthesis and Characterization of a Fe3O4@PNIPAM-Chitosan Nanocomposite and Its Potential Application in Vincristine Delivery. Polymers 2021, 13, 1704. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.H.; Choi, H.W.; Lim, J.H.; Kim, J.W.; Chung, B.G. Near-Infrared Light-Triggered Thermo-responsive Poly(N-isopropylacrylamide)-Pyrrole Nanocomposites for Chemo-photothermal Cancer Therapy. Nanoscale Res. Lett. 2020, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Atoufi, Z.; Kamrava, S.K.; Davachi, S.M.; Hassanabadi, M.; Garakani, S.S.; Alizadeh, R.; Farhadi, M.; Tavakol, S.; Bagher, Z.; Motlagh, G.H. Injectable PNIPAM/Hyaluronic acid hydrogels containing multipurpose modified particles for cartilage tissue engineering: Synthesis, characterization, drug release and cell culture study. Int. J. Biol. Macromol. 2019, 139, 1168–1181. [Google Scholar] [CrossRef]
- Parameswaran-Thankam, A.; Parnell, C.M.; Watanabe, F.; RanguMagar, A.B.; Chhetri, B.P.; Szwedo, P.K.; Biris, A.S.; Ghosh, A. Guar-based injectable thermoresponsive hydrogel as a scaffold for bone cell growth and controlled drug delivery. ACS Omega 2018, 3, 15158–15167. [Google Scholar] [CrossRef]
- Liu, L.; Zeng, J.; Zhao, X.; Tian, K.; Liu, P. Independent temperature and pH dual-responsive PMAA/PNIPAM microgels as drug delivery system: Effect of swelling behavior of the core and shell materials in fabrication process. Colloids Surf. A Physicochem. Eng. Asp. 2017, 526, 48–55. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. PNIPAM-b-PDMAEA double stimuli responsive copolymers: Effects of composition, end groups and chemical modification on solution self-assembly. Eur. Polym. J. 2020, 135, 109867. [Google Scholar] [CrossRef]
- Rasib, S.Z.M.; Akil, H.M.; Yahya, A. Effect of different composition on particle size chitosan-PMAA-PNIPAM hydrogel. Proc. Chem. 2016, 19, 388–393. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.L.; Zhang, X.Y.; Fu, J.Y.; Xu, F.; Chen, Y.S. Novel Temperature and pH Dual Sensitive PNIPAM/CMCS/MWCNTs semi-IPN Nanohybrid Hydrogels: Synthesis, Characterization and DOX Drug Release. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 398–409. [Google Scholar] [CrossRef]
- Ohnsorg, M.L.; Ting, J.M.; Jones, S.D.; Jung, S.; Bates, F.S.; Reineke, T.M. Tuning PNIPAm self-assembly and thermoresponse: Roles of hydrophobic end-groups and hydrophilic comonomer. Polym. Chem. 2019, 10, 3469–3479. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Liu, Z.Q.; Luo, Y.L.; Xu, F.; Chen, Y.S. Tristimuli responsive carbon nanotubes covered by mesoporous silica graft copolymer multifunctional materials for intracellular drug delivery. J. Ind. Eng. Chem. 2019, 80, 431–443. [Google Scholar] [CrossRef]
- Zhang, K.; Li, F.; Wu, Y.; Feng, L.; Zhang, L. Construction of ionic thermo-responsive PNIPAM/γ-PGA/PEG hydrogel as a draw agent for enhanced forward-osmosis desalination. Desalination 2020, 495, 114667. [Google Scholar] [CrossRef]
- Kanidi, M.; Papagiannopoulos, A.; Matei, A.; Dinescu, M.; Pispas, S.; Kandyla, M. Functional surfaces of laser-microstructured silicon coated with thermoresponsive PS/PNIPAM polymer blends: Switching reversibly between hydrophilicity and hydrophobicity. Appl. Surf. Sci. 2020, 527, 146841. [Google Scholar] [CrossRef]
- Zhao, X.; Shan, G. PSMA-b-PNIPAM copolymer micelles with both a hydrophobic segment and a hydrophilic terminal group: Synthesis, micelle formation, and characterization. Colloid Polym. Sci. 2019, 297, 1353–1363. [Google Scholar] [CrossRef]
- Lostalé-Seijo, I.; Montenegro, J. Synthetic materials at the forefront of gene delivery. Nat. Rev. Chem. 2018, 2, 258–277. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jesson, K.; Wei, Z.Z.; Logun, M.; Lenear, C.; Tan, S.; Gu, X.; Jiang, M.Q.; Karumbaiah, L.; Yu, S.P.; et al. Cortical Transplantation of Brain-Mimetic Glycosaminoglycan Scaffolds and Neural Progenitor Cells Promotes Vascular Regeneration and Functional Recovery after Ischemic Stroke in Mice. Adv. Healthc. Mater. 2020, 9, 1900285. [Google Scholar] [CrossRef]
- Cui, Z.; Lee, B.H.; Pauken, C.; Vernon, B.L. Degradation, cytotoxicity, and biocompatibility of NIPAAm-based thermosensitive, injectable, and bioresorbable polymer hydrogels. J. Biomed. Mater. Res. Part A 2011, 98, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krebs, M.D.; Salter, E.; Chen, E.; Sutter, K.A.; Alsberg, E. Calcium phosphate-DNA nanoparticle gene delivery from alginate hydrogels induces in vivo osteogenesis. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2010, 92, 1131–1138. [Google Scholar]
- Vijayavenkataraman, S.; Yan, W.-C.; Lu, W.F.; Wang, C.-H.; Fuh, J.Y. 3D bioprinting of tissues and organs for regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 296–332. [Google Scholar] [CrossRef]
- Singh, S.; Choudhury, D.; Yu, F.; Mironov, V.; Naing, M.W. In situ bioprinting–Bioprinting from benchside to bedside? Acta Biomater. 2020, 101, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 20175, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sood, N.; Bhardwaj, A.; Mehta, S.; Mehta, A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016, 23, 748–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Yu, J.; Ren, K.; Zuo, J.; Ding, J.; Chen, X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules 2019, 20, 1478–1492. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Guo, Y.; Zhao, X.; Du, T.; Zhu, J.; Xie, Y.; Wu, F.; Wang, Y.; Guan, M. Poly (N-isopropylacrylamide) Based Electrically Conductive Hydrogels and Their Applications. Gels 2022, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Singh, S.R.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Park, J.S.; Yang, H.N.; Woo, D.G.; Jeon, S.Y.; Park, K.-H. Poly (N-isopropylacrylamide-co-acrylic acid) nanogels for tracing and delivering genes to human mesenchymal stem cells. Biomaterials 2013, 34, 8819–8834. [Google Scholar] [CrossRef]
- Zhang, J.T.; Petersen, S.; Thunga, M.; Leipold, E.; Weidisch, R.; Liu, X.; Fahr, A.; Jandt, K.D. Micro-structured smart hydrogels with enhanced protein loading and release efficiency. Acta Biomater. 2010, 6, 1297–1306. [Google Scholar] [CrossRef]
- Chalanqui, M.J.; Pentlavalli, S.; McCrudden, C.; Chambers, P.; Ziminska, M.; Dunne, N.; McCarthy, H.O. Influence of alginate backbone on efficacy of thermo-responsive alginate-g-PNIPAM hydrogel as a vehicle for sustained and controlled gene delivery. Mater. Sci. Eng. C 2019, 95, 409–421. [Google Scholar] [CrossRef]
- Ghaeini-Hesaroeiye, S.; Bagtash, H.R.; Boddohi, S.; Vasheghani-Farahani, E.; Jabbari, E. Thermoresponsive Nanogels Based on Different Polymeric Moieties for Biomedical Applications. Gels 2020, 6, 20. [Google Scholar] [CrossRef]
- Kandil, R.; Merkel, O.M. Recent progress of polymeric nanogels for gene delivery. Curr. Opin. Colloid Interface Sci. 2019, 39, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Fliervoet, L.A.L.; van Nostrum, C.F.; Hennink, W.E.; Vermonden, T. Balancing hydrophobic and electrostatic interactions in thermosensitive polyplexes for nucleic acid delivery. Multifunct. Mater. 2019, 2, 024002. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lan, Y.H.; Chuang, C.C.; Lu, W.T.; Chan, L.Y.; Hsu, P.W.; Chen, J.P. Injectable thermo-sensitive chitosan hydrogel containing CPT-11-loaded EGFR-targeted graphene oxide and SLP2 shRNA for localized drug/gene delivery in glioblastoma therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef] [PubMed]
- Fliervoet, L.A.; Zhang, H.; van Groesen, E.; Fortuin, K.; Duin, N.J.; Remaut, K.; Schiffelers, R.M.; Hennink, W.E.; Vermonden, T. Local release of siRNA using polyplex-loaded thermosensitive hydrogels. Nanoscale 2020, 12, 10347–10360. [Google Scholar] [CrossRef] [PubMed]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and evaluation of a thermoresponsive degradable chitosan-grafted PNIPAAm hydrogel as a ‘smart’ gene delivery system. Materials 2020, 13, 2530. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Liu, Z.; Yuan, P.; Jin, R.; Wang, X.; Jiang, T.; Chen, X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J. Mater. Chem. B 2019, 7, 2722–2735. [Google Scholar] [CrossRef]
- Yang, H.Y.; van Ee, R.J.; Timmer, K.; Craenmehr, E.G.; Huang, J.H.; Öner, F.C.; Dhert, W.J.; Kragten, A.H.; Willems, N.; Grinwis, G.C.; et al. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015, 23, 214–228. [Google Scholar] [CrossRef]
- Mansour, J.M. Biomechanics of cartilage. Kinesiol. Mech. Pathomechanics Hum. Mov. 2003, 2, 69–83. [Google Scholar]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef]
- Keeney, J.A.; Eunice, S.; Pashos, G.; Wright, R.W.; Clohisy, J.C. What is the evidence for total knee arthroplasty in young patients?: A systematic review of the literature. Clin. Orthop. Relat. Res. 2011, 469, 574–583. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.A.; Chen, R.; van Veen, T.; Bryan, N. Hydrogels for tissue engineering and regenerative medicine. J. Mater. Chem. B. 2014, 2, 5319–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yun, S.; Du, Y.; Zannettino, A.C.W.; Zhang, H. Fabrication of a cartilage patch by fusing hydrogel-derived cell aggregates onto electrospun film. Tissue Eng. Part A 2020, 26, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Means, A.K.; Shrode, C.S.; Whitney, L.V.; Ehrhardt, D.A.; Grunlan, M.A. Double network hydrogels that mimic the modulus, strength, and lubricity of cartilage. Biomacromolecules 2019, 20, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Eswaramoorthy, R.; Lin, T.H.; Chen, C.H.; Fu, Y.C.; Wang, C.K.; Wu, S.C.; Wang, G.J.; Chang, J.K.; Ho, M.L. Enhancement of chondrogenesis of adipose-derived stem cells in HA-PNIPAAm-CL hydrogel for cartilage regeneration in rabbits. Sci. Rep. 2018, 8, 10526. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Bai, R.; Suo, Z. Topological adhesion of wet materials. Adv. Mater. 2018, 30, 1800671. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef]
- Mellati, A.; Fan, C.M.; Tamayol, A.; Annabi, N.; Dai, S.; Bi, J.; Jin, B.; Xian, C.; Khademhosseini, A.; Zhang, H. Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering. Biotechnol. Bioeng. 2017, 114, 217–231. [Google Scholar] [CrossRef] [Green Version]
- Brunelle, A.R.; Horner, C.B.; Low, K.; Ico, G.; Nam, J. Electrospun thermosensitive hydrogel scaffold for enhanced chondrogenesis of human mesenchymal stem cells. Acta Biomater. 2018, 66, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Saghebasl, S.; Davaran, S.; Rahbarghazi, R.; Montaseri, A.; Salehi, R.; Ramazani, A. Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering. Polym. Ed. 2018, 29, 1185–1206. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable Chitosan/κ-Carrageenan Hydrogel Designed with au Nanoparticles: A Conductive Scaffold for Tissue Engineering Demands; Elsevier: Amsterdam, The Netherlands, 2019; Volume 126. [Google Scholar] [CrossRef]
- Mohamed, A.M.F.S. An overview of bone cells and their regulating factors of differentiation. Malays. J. Med. Sci. 2008, 15, 4–12. [Google Scholar]
- Park, J.-B. The use of hydrogels in bone-tissue engineering. Biomater. Bioeng. Dent. 2011, 16, e115–e118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adibfar, A.; Amoabediny, G.; Eslaminejad, M.B.; Mohamadi, J.; Bagheri, F.; Doulabi, B.Z. VEGF delivery by smart polymeric PNIPAM nanoparticles affects both osteogenic and angiogenic capacities of human bone marrow stem cells. Mater. Sci. Eng. C 2018, 93, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Urban, B.; Reis, B.; Yu, X.; Grab, A.L.; Cavalcanti-Adam, E.A.; Kuckling, D. Switchable Release of Bone Morphogenetic Protein from Thermoresponsive Poly (NIPAM-co-DMAEMA)/Cellulose Sulfate Particle Coatings. Polymers 2018, 10, 1314. [Google Scholar] [CrossRef] [Green Version]
- Prasadh, S.; Suresh, S.; Wong, R. Osteogenic potential of graphene in bone tissue engineering scaffolds. Materials 2018, 11, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorpe, A.A.; Creasey, S.; Sammon, C.; le Maitre, C.L. Hydroxyapatite nanoparticle injectable hydrogel scaffold to support osteogenic differentiation of human mesenchymal stem cells. Eur. Cells Mater. 2016, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.A.; Freeman, C.; Farthing, P.; Callaghan, J.; Hatton, P.V.; Brook, I.M.; Sammon, C.; Le Maitre, C.L. In vivo safety and efficacy testing of a thermally triggered injectable hydrogel scaffold for bone regeneration and augmentation in a rat model. Oncotarget 2018, 9, 18277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic. Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Oberbek, P.; Bolek, T.; Chlanda, A.; Hirano, S.; Kusnieruk, S.; Rogowska-Tylman, J.; Nechyporenko, G.; Zinchenko, V.; Swieszkowski, W.; Puzyn, T. Characterization and influence of hydroxyapatite nanopowders on living cells. Beilstein J. Nanotechnol. 2018, 9, 3079–3094. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Sun, J.; Tan, L.; Yan, Q.; Li, L.; Chen, L.; Liu, X.; Bin, S. Enhanced osteogenesis and therapy of osteoporosis using simvastatin loaded hybrid system. Bioact. Mater. 2020, 5, 348–357. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, P.; Shi, X.; Ling, T.; Zeng, W.; Chen, A.; Yang, W.; Zhou, Z. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repair. ACS Nano 2019, 13, 9595–9606. [Google Scholar] [CrossRef]
- Petre, D.G.; Nadar, R.; Tu, Y.; Paknahad, A.; Wilson, D.A.; Leeuwenburgh, S.C. Thermoresponsive brushes facilitate effective reinforcement of calcium phosphate cements. ACS Appl. Mater. Interfaces 2019, 11, 26690–26703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, C.A.; Martins, M.V.S.; Bressiani, A.H.; Bressiani, J.C.; Leyva, M.E.; de Queiroz, A.A.A. Electrochemical preparation and characterization of PNIPAM-HAp scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2017, 81, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-F.; Zeng, Q.; Wang, H.; Hao, Y. Preparation and swelling behavior of pH/temperature responsive semi-IPN hydrogel based on carboxymethyl xylan and poly(N-isopropyl acrylamide). Cellulose 2018, 26, 1909–1922. [Google Scholar] [CrossRef]
- Shumer, D.E.; Natalie, J.; Nokoff, N.P.S. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Mihalko, E.; Huang, K.; Sproul, E.; Cheng, K.; Brown, A.C. Targeted treatment of ischemic and fibrotic complications of myocardial infarction using a dual-delivery microgel therapeutic. ACS Nano 2018, 12, 7826–7837. [Google Scholar] [CrossRef]
- Lee, D.J.; Cavasin, M.A.; Rocker, A.J.; Soranno, D.E.; Meng, X.; Shandas, R.; Park, D. An injectable sulfonated reversible thermal gel for therapeutic angiogenesis to protect cardiac function after a myocardial infarction. J. Biol. Eng. 2019, 13, 6. [Google Scholar] [CrossRef]
- Rocker, A.J.; Lee, D.J.; Shandas, R.; Park, D. Injectable Polymeric Delivery System for Spatiotemporal and Sequential Release of Therapeutic Proteins To Promote Therapeutic Angiogenesis and Reduce Inflammation. ACS Biomater. Sci. Eng. 2020, 6, 1217–1227. [Google Scholar] [CrossRef]
- Cui, X.; Tang, J.; Hartanto, Y.; Zhang, J.; Bi, J.; Dai, S.; Qiao, S.Z.; Cheng, K.; Zhang, H. NIPAM-based microgel microenvironment regulates the therapeutic function of cardiac stromal cells. ACS Appl. Mater. Interfaces 2018, 10, 37783–37796. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, S.; Liu, Q.; Xiu, K.; Lei, I.; Wang, Z.; Ma, P.X. Biodegradable Nanofibrous Temperature-Responsive Gelling Microspheres for Heart Regeneration. Adv. Funct. Mater. 2020, 30, 2000776. [Google Scholar] [CrossRef]
- Navaei, A.; Truong, D.; Heffernan, J.; Cutts, J.; Brafman, D.; Sirianni, R.W.; Vernon, B.; Nikkhah, M. PNIPAAm-based biohybrid injectable hydrogel for cardiac tissue engineering. Acta Biomater. 2016, 32, 10–23. [Google Scholar] [CrossRef]
- Breslin, J.W.; Yang, Y.; Scallan, J.P.; Sweat, R.S.; Adderley, S.P.; Murfee, W.L. Lymphatic vessel network structure and physiology. Compr. Physiol. 2019, 9, 207–299. [Google Scholar] [CrossRef]
- Wu, C.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra, H.; Cordova, M.; Chen, C.S.J.; Rajadhyaksha, M. Confocal imaging-guided laser ablation of basal cell carcinomas: An ex vivo study. J. Investig. Dermatol. 2015, 135, 612–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.; Lee, J. Thermoresponsive Inverted Colloidal Crystal Hydrogel Scaffolds for Lymphoid Tissue Engineering. Adv. Healthc. Mater. 2020, 9, 1901556. [Google Scholar] [CrossRef]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly (N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef]
- Dosh, R.H.; Essa, A.; Jordan-Mahy, N.; Sammon, C.; le Maitre, C.L. Use of hydrogel scaffolds to develop an in vitro 3D culture model of human intestinal epithelium. Acta Biomater. 2017, 62, 128–143. [Google Scholar] [CrossRef]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; le Maitre, C.L. Long-term in vitro 3D hydrogel co-culture model of inflammatory bowel disease. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Dosh, R.H.; Jordan-Mahy, N.; Sammon, C.; le Maitre, C.L. Use of L-pNIPAM hydrogel as a 3D-scaffold for intestinal crypts and stem cell tissue engineering. Biomater. Sci. 2019, 7, 4310–4324. [Google Scholar] [CrossRef]
- Mellati, A.; Valizadeh, M.; Madani, S.H.; Dai, S.; Bi, J. Poly (N-isopropylacrylamide ) hydrogel/chitosan scaffold hybrid for three-dimensional stem cell culture and cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2016, 104, 2764–2774. [Google Scholar] [CrossRef]
- Amiryaghoubi, N.; Pesyan, N.N.; Fathi, M.; Omidi, Y. Injectable thermosensitive hybrid hydrogel containing graphene oxide and chitosan as dental pulp stem cells scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 162, 1338–1357. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and Highly Flexible Hydrogels Capable of Stimulating Cardiac Differentiation of Cardiosphere-Derived Cells under Static and Dynamic Mechanical Training Conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhou, J.; Liu, Z.; Chen, J.; Lü, S.; Sun, H.; Li, J.; Lin, Q.; Yang, B.; Duan, C.; et al. A PNIPAAm-based thermosensitive hydrogel containing SWCNTs for stem cell transplantation in myocardial repair. Biomaterials 2014, 35, 5679–5688. [Google Scholar] [CrossRef] [PubMed]
- Patra, P.; Rameshbabu, A.P.; Das, D.; Dhara, S.; Panda, A.B.; Pal, S. Stimuli-responsive, biocompatible hydrogel derived from glycogen and poly(: N -isopropylacrylamide) for colon targeted delivery of ornidazole and 5-amino salicylic acid. Polym. Chem. 2016, 7, 5426–5435. [Google Scholar] [CrossRef]
- Hoang, H.T.; Jo, S.H.; Phan, Q.T.; Park, H.; Park, S.H.; Oh, C.W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using ‘click’ chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Zhu, J.; Han, H.; Zhang, J.Z.; Wu, F.F.; Qin, X.H.; Yu, J.Y. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2018, 65, 305–316. [Google Scholar] [CrossRef]
- Dong, Y.; Zhuang, H.; Hao, Y.; Zhang, L.; Yang, Q.; Liu, Y.; Qi, C.; Wang, S. Poly(N-isopropyl-acrylamide)/poly(γ-glutamic acid) thermo-sensitive hydrogels loaded with superoxide dismutase for wound dressing application. Int. J. Nanomed. 2020, 15, 1939–1950. [Google Scholar] [CrossRef] [Green Version]
- Hathaway, H.; Alves, D.R.; Bean, J.; Esteban, P.P.; Ouadi, K.; Sutton, J.M.; Jenkins, A.T.A. Poly (N-isopropylacrylamide-co-allylamine)(PNIPAM-co-ALA) nanospheres for the thermally triggered release of Bacteriophage K. Eur. J. Pharm. Biopharm. 2015, 96, 437–441. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Yang, Y.; Zhang, L.; Xu, K.; Wang, J. Study of thermal-sensitive alginate-Ca2+/poly (N-isopropylacrylamide) hydrogels supported by cotton fabric for wound dressing applications. Text. Res. J. 2019, 89, 801–813. [Google Scholar] [CrossRef]
- Qasim, M.; Udomluck, N.; Chang, J.; Park, H.; Kim, K. Antimicrobial activity of silver nanoparticles encapsulated in poly-N-isopropylacrylamide-based polymeric nanoparticles. Int. J. Nanomed. 2018, 13, 235. [Google Scholar] [CrossRef] [Green Version]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, H.; Min, D.; Miao, X.; Li, F.; Dong, L.; Xing, J.; Guo, G.; Wang, X. Dual layered wound dressing with simultaneous temperature {\&} antibacterial regulation properties. Mater. Sci. Eng. C 2019, 94, 1077–1082. [Google Scholar] [CrossRef]
- Blacklow, S.O.; Li, J.; Freedman, B.R.; Zeidi, M.; Chen, C.; Mooney, D.J. Bioinspired mechanically active adhesive dressings to accelerate wound closure. Sci. Adv. 2019, 5, eaaw3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, A.; Sharma, Y.; Hattori, S.; Terada, D.; Sharma, A.K.; Turner, A.P.; Kobayashi, H. Influence of poly(N-isopropylacrylamide)-CNT-polyaniline three-dimensional electrospun microfabric scaffolds on cell growth and viability. Biopolymers 2013, 99, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagherifard, S.; Tamayol, A.; Mostafalu, P.; Akbari, M.; Comotto, M.; Annabi, N.; Ghaderi, M.; Sonkusale, S.; Dokmeci, M.R.; Khademhosseini, A. Dermal Patch with Integrated Flexible Heater for on Demand Drug Delivery. Adv. Healthc. Mater. 2016, 5, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Wang, Y.; Zhang, H.; Zhang, H.; Dong, W.; Sun, W.; Zhao, Y. Responsive and self-healing structural color supramolecular hydrogel patch for diabetic wound treatment. Bioact. Mater. 2022, 15, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-W.; Hsu, C.-C.; Liu, Y.-Z.; Wei, P.-L.; Chen, J.-K. Supermolecules of poly(N-isopropylacrylamide) complexating Herring sperm DNA with bio-multiple hydrogen bonding. Colloids Surf. B Biointerfaces 2016, 148, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-W.; Lee, A.-W.; Huang, C.-H.; Chen, J.-K. Characterization of poly(N-isopropylacrylamide)–nucleobase supramolecular complexes featuring bio-multiple hydrogen bonds. Soft. Matter. 2014, 10, 8330–8340. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef]
- Jin, X.; Jiang, H.; Li, G.; Fu, B.; Bao, X.; Wang, Z.; Hu, Q. Stretchable, conductive PAni-PAAm-GOCS hydrogels with excellent mechanical strength, strain sensitivity and skin affinity. Chem. Eng. J. 2020, 394, 124901. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, Injectable, Self-Healing Conductive Hydrogel Enabled by Multiple Hydrogen Bonding toward Wearable Electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Wu, T.; Cui, C.; Huang, Y.; Liu, Y.; Fan, C.; Han, X.; Yang, Y.; Xu, Z.; Liu, B.; Fan, G.; et al. Coadministration of an Adhesive Conductive Hydrogel Patch and an Injectable Hydrogel to Treat Myocardial Infarction. ACS Appl. Mater. Interfaces 2020, 12, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Wang, H.; Ma, P.X.; Guo, B. Self-healing conductive hydrogels: Preparation, properties and applications. Nanoscale 2020, 12, 1224–1246. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.S.; Narasimhan, B.N.; Travas-Sejdic, J.; Malmström, J. Soft conducting polymer polypyrrole actuation based on poly(N-isopropylacrylamide) hydrogels. Sens. Actuators B Chem. 2021, 343, 130167. [Google Scholar] [CrossRef]
- Zhou, Y.; Wan, C.; Yang, Y.; Yang, H.; Wang, S.; Dai, Z.; Ji, K.; Jiang, H.; Chen, X.; Long, Y. Highly Stretchable, Elastic, and Ionic Conductive Hydrogel for Artificial Soft Electronics. Adv. Funct. Mater. 2019, 29, 1806220. [Google Scholar] [CrossRef]
- Green, R. Elastic and conductive hydrogel electrodes. Nat. Biomed. Eng. 2019, 3, 9–10. [Google Scholar] [CrossRef]
- Deng, Z.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Sui, X.; Guo, H.; Cai, C.; Li, Q.; Wen, C.; Zhang, X.; Wang, X.; Yang, J.; Zhang, L. Ionic conductive hydrogels with long-lasting antifreezing, water retention and self-regeneration abilities. Chem. Eng. J. 2021, 419, 129478. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, T.; Zhang, S.; Zhang, P.; Li, R.; Ma, N.; Wei, H.; Zhang, X. Conductive and Tough Smart Poly( N -isopropylacrylamide) Hydrogels Hybridized by Green Deep Eutectic Solvent. Macromol. Chem. Phys. 2021, 222, 2000301. [Google Scholar] [CrossRef]
- Han, L.; Liu, K.; Wang, M.; Wang, K.; Fang, L.; Chen, H.; Zhou, J.; Lu, X. Mussel-Inspired Adhesive and Conductive Hydrogel with Long-Lasting Moisture and Extreme Temperature Tolerance. Adv. Funct. Mater. 2018, 28, 1704195. [Google Scholar] [CrossRef]
- Park, J.; Jeon, J.; Kim, B.; Lee, M.S.; Park, S.; Lim, J.; Yi, J.; Lee, H.; Yang, H.S.; Lee, J.Y. Electrically Conductive Hydrogel Nerve Guidance Conduits for Peripheral Nerve Regeneration. Adv. Funct. Mater. 2020, 30, 2003759. [Google Scholar] [CrossRef]
- Jo, H.; Sim, M.; Kim, S.; Yang, S.; Yoo, Y.; Park, J.H.; Yoon, T.H.; Kim, M.G.; Lee, J.Y. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017, 48, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Farajollahi, M.; Choi, Y.S.; Lin, I.T.; Marshall, J.E.; Thompson, N.M.; Kar-Narayan, S.; Madden, J.D.W.; Smoukov, S.K. Electroactive polymers for sensing. Interface Focus. 2016, 6, 20160026. [Google Scholar] [CrossRef]
- Khan, A.; Alamry, K.A.; Jain, R.K. Polypyrrole nanoparticles-based soft actuator for artificial muscle applications. RSC Adv. 2019, 9, 39721–39734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ren, X.; Fan, H.; Ma, J.; Wang, C.; Zhang, M.; Zhao, N. Hierarchical Co3O4/PANI hollow nanocages: Synthesis and application for electrode materials of supercapacitors. Appl. Surf. Sci. 2018, 441, 194–203. [Google Scholar] [CrossRef]
- Harjo, M.; Järvekülg, M.; Tamm, T.; Otero, T.F.; Kiefer, R. Concept of an artificial muscle design on polypyrrole nanofiber scaffolds. PLoS ONE 2020, 15, e0232851. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Xue, Y.; Xue, Y.; Zhang, J.; Chen, X.; Zhang, J.; Chen, G.; Zhang, K.; Lin, J.; Guo, C.; Liu, J. Trigger-Detachable Hydrogel Adhesives for Bioelectronic Interfaces. Adv. Funct. Mater. 2021, 31, 2106446. [Google Scholar] [CrossRef]






| Hydrogel Composition | Drug | Preparation Technique | Key Features | References |
|---|---|---|---|---|
| PNIPAM/poly (ε-caprolactone) 8 dimethacrylate (PCLDMA)/bisacryloylcystamine (BACy) | Levofloxacin | Polymerization | The thermosensitive and biodegradable hydrogels were made from PCLDMA as a hydrolytically degradable unit along with a hydrophobic unit, with BACy as reducible degradation combined with a hydrophilic unit. The advantages of both thermoresponsive and biodegradable polymer systems was amalgamated. | [38] |
| PNIPAM/PPCN | Chemokine SDF -1 alpha | Sequential polycondensation and radical polymerization | The thermosensitive and biodegradable hydrogels with inherent antioxidant properties for the effective distribution of therapeutics was observed. | [110] |
| PNIPAM/Hyaluronic acid (HA) | Melatonin | Copolymerization | PNIPAM with HA increased the interrelatedness in microscopic structure with mechanical and chemical properties, and the hydrogels were highly adaptable to liquid/gel conversion temperatures which aid the improved support of the microenvironment for cell expansions and aggregates. | [188] |
| PNIPAM/Hydroxypropyl guar-graft-poly(N-vinyl caprolactam) | Ciprofloxacin | Graft polymerization | In situ covalent crosslinking of HPG-g-PNVCL copolymer with nano-hydroxyapatite (n-HA) by using divinyl sulfone (DVS) as a crosslinking agent to achieve HPG-g-PNVCL/n-HA/DVS composite material. | [189] |
| PNIPAM/poly(methacrylic acid) | Doxorubicin | Distillation precipitation or emulsion precipitation copolymerization | The PMAA/PNIPAM-1 microgel, prepared with the moderately-swollen PMAA cores for thicker PNIPAM shells via distillation precipitation copolymerization in acetonitrile, displayed more efficient pH and temperature-independent dual-stimuli responsive controlled releasing performance, while the PMAA/PNIPAM-2 microgels prepared with the fully swollen PMAA cores for thicker PNIPAM shells, via emulsion precipitation copolymerization in water, influenced higher drug-loading capability. | [190] |
| PNIPAM/HOOC-PNIPAM)-b-poly(2-(dimethylamino) ethyl acrylate)-C12H25 (HOOC-PNIPAM-b-PDMAEA-C12H25)/HOOC-poly(2-dimethylamino)ethyl acrylate)-b-PNIPAM)-C12H25 (HOOC-PDMAEA-b-PNIPAM-C12H25) | - | Sequential reversible addition-fragmentation chain transfer (RAFT) polymerization | The diblock copolymers were chemically modified to strong cationic, double hydrophilic, block polyelectrolytes via quaternization reaction on the PDMAEA block. The quaternized block copolymers form larger aggregates than the amine-based block copolymers because of the electrostatic repulsions of the positively charged quaternary amine groups. | [191] |
| PNIPAM/chitosan-poly(methacrylic acid) Cs-PMAA | - | Free radical emulsion polymerization | Copolymerized chitosan with MAA along with NIPAM is an improved version of chitosan gel to be further receptive to the atmosphere of the human body, including different pH, ionic strength, temperature, electric field, and enzyme activities. The small size of the particles is essential to ensure that the particles get through to the target site, especially in drug delivery. | [192] |
| PNIPAM/carboxymethyl chitosan/multiwalled carbon nanotube | Doxorubicin | In situ crosslinking polymerization | The hydrogels demonstrated dual-responsiveness of pH and temperature, and high maximal swelling ratios were possessed by multiwalled carbon nanotubes (MWCNTs)–COOH. The hydrogel could be utilized for the site-specific direct delivery of protein or hydrophilic anticancer drugs. | [193] |
| PNIPAM/3-(methacryloxypropyl)trimethoxysilane) | ibuprofen | Grafting and polymerization | The hybrid nanoparticles were monodispersed in an aqueous medium and displayed temperature dependency of standard hydrodynamic diameter, promoting them as drug nanocarriers. They demonstrated the exceptional temperature-regulated delivery of the model drug. Specifically, a low % release of ibuprofen below LCST along with a complete and fast ibuprofen delivery at higher than LCST contrasted the earlier report. | [177] |
| PNIPAM/poly(2-(dimethylamino) ethyl acrylate)20-b-PNIPAM)11-b-poly(oligo ethylene glycol methyl ether acrylate)18 (PDMAEA20-b-PNIPAM11-b-POEGA18) | - | Sequential reversible addition-fragmentation chain transfer polymerization | The thermoresponsive behavior was displayed by amine-based triblock terpolymer, despite the low amount of PNIPAM block in comparison to other comprising blocks. The chemically altered triblock terpolymers self-assemble into larger aggregates in the whole temperature scale compared to the amine-functionalized triblock terpolymer as a result of electrostatic repulsions of the permanently charged quaternary amine groups of the modified PDMAEA blocks. | [191] |
| PNIPAM/N,N-dimethylacrylamide (DMA) | - | Reversible addition-fragmentation chain transfer (RAFT) polymerization | The synthesis of six NIPAM and DMA-based statistical, ABA triblock, and ABABA pentablock copolymers for each comprised one or two dodecyl hydrocarbon end-groups. The results demonstrated extraordinary and carefully balanced tradeoffs among short non-polar end groups and customized hydrophobicity in the nanoscale self-fabrication of PNIPAm-based copolymers in the water near the LCST. | [194] |
| PNIPAM/poly(2-(4-formylbenzoyloxy) ethyl methacrylate) | Doxorubicin | Disulfide linkages | Shells of disulfide-bonded temperature-sensitive block copolymers act as gatekeepers to control drug release. The developed multifunctional materials do not produce premature release in blood circulation but accelerate drug release inside cancer cells. | [195] |
| PNIPAM/polyglutamic acid (γ-PGA)/polyethylene glycol (PEG) | - | Polymerization | The optimal mass ratio of comonomers (NIPAM, γ-PGA, and PEG), crosslinker, and initiator was secured at 1:0.2:1:0.01:0.01, defined by the response surface method (RSM). It was also discovered by RSM that the ESR was considerably reliant on the crosslinker along with the collaboration amongst the initiator and γ-PGA. | [196] |
| PNIPAM/polystyrene (PS) | - | Anionic polymerization | Thermoresponsive wetting performance as a role of substrate micromorphology with the surface. PS/PNIPAM films of various fusions were spin-casted on microstructured silicon substrates together with or devoid of a native SiO2 layer, and take up the benefit of the large specific area of the silicon substrates to enrich the film thermoresponsiveness. | [197] |
| PNIPAM/poly (stearyl methacrylate) | - | Reversible addition-fragmentation chain-transfer (RAFT) polymerization | The triblock copolymer micelles demonstrated a distinctive evolution, initially developing into small, then developing into larger, and finally stable. The transition process was fast as well as reversible with temperature. The hydrophobic PSMA chain segment dropped the LCST of the diblock copolymer micelles. | [198] |
| Device | Model Drug | Composition | Preparation Technique | Applications | Results | References |
|---|---|---|---|---|---|---|
| Hydrogels | RALA, plasmid DNA (p-DNA) | Alginate (Alg) grafted PNIPAM (Alg-g-PNIPAM) | Free radical polymerization | Castrate-resistant prostate cancer (CRPC) | The copolymer’s alginate backbone significantly influenced the mechanical and structural properties of hydrogels. At 37 °C, high-pitched MW alginate improved the copolymer’s rigidity, and the M/G ratio affected rigidity as well as the molecular network. In contrast to uncomplexed pDNA, which had a significant rupture release during the first six hours in Alg-g-PNIPAM hydrogels, RALA/pDNA NPs had a prolonged and controlled release over time. This offers up a slew of possibilities for remedial pDNA delivery from this thermoresponsive hydrogel, which proved to have a wide range of medical applications. | [212] |
| Thermoresponsive hydrogel | RALA/pEGFP–N1 | Chitosan-g-PNIPAM crosslinked with genipin | Free radical polymerization | - | The proportion of chitosan in the copolymer affected the hydrogel’s breakdown, swelling, NP release level, and storage modulus. The Cs-g-injectability PNIPAM’s at room temperature suggested that it may be delivered to the target site in a minimally invasive manner. The hydrogel’s ability to provide long-acting drugs to target tissues was demonstrated by sustained NP release and breakdown over three weeks. More crucially, the nucleic acid payload remained active, as evidenced by the NCTC-929 fibroblast cell line’s excellent transfection. | [218] |
| Thermosensitive hydrogel | Irinotecan (CPT-11)/cetuximab (CET) conjugate graphene oxide (GO) (GO-CET/CPT11), stomatin like protein 2 (SLP2), and short heparin RNA (shRNA) | Chitosan-g-PNIPAM (CPN) | Free radical polymerization | Glioblastoma multiforme | Controlled drug release and increased mechanical strength of the in situ-produced hydrogel were achieved by combining a negatively charged nanocarrier and a positively charged CPN. CPT-11 release from a drug-loaded hydrogel exhibited a 28-day continuous release pattern, whereas the intricate shear modulus rose fivefold after entrapping GO-CET in the hydrogel. The formulation increased anti-tumor activity in vitro by eliciting a 53% apoptotic rate in 2 days. A xenograft tumor model was used to illustrate treatment efficacy, with a 40% reduction in tumor size after 12 days compared to the untreated control group. | [216] |
| Thermosensitive mesoporous silica nanoparticles (MSN) | microRNA-222 and aspirin (ASP) | Poly(ethylene glycol)-b-poly(lactic-co-glycolic acid)-b-PNIPAM (PEG-PLGA-PNIPAM) | Atom transfer radical polymerization and ring-opening copolymerization | Bone tissue engineering | As previously reported, ASP stimulated bone production, and miR222 triggered Wnt/-catenin/nemo-like kinase signaling to drive differentiation of bone mesenchymal stem cells to neural-like cells. Injection of co-delivered MSN hydrogel into a rat mandibular bone defect resulted in neurogenesis and faster bone development, suggesting that the injectable ASP and miR222co-delivering colloid hydrogel has potential for vascularized BTE. | [219] |
| Thermoresponsive hydrogels | SiRNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | PNIPAM/MgAl-layered double hydroxides (LDHs) (MgAl-LDH) | Radical polymerization | Degenerative disease of cartilaginous tissues | When the temperature of the hybrid hydrogel was increased from 25 to 37 degrees Celsius, it transitioned from a fluid to viscous gel phase in less than 10 s. The introduction of siRNA against a housekeeping gene into an in vitro model of cartilaginous tissue degeneration comprised of osteoarthritic cells was reported to achieve gene silencing in situ for 6 days with a high gene silencing efficacy (>80%). Providing extracellular matrix scaffolds and interfering with degenerative factor expression, therapeutic RNA oligonucleotides with supporting hydrogel material may offer promises in treating cartilaginous tissue degeneration. | [220] |
| Nanogels | Green fluorescence protein (GFP) gene, amine functional magnetic iron oxide nanoparticles (NH2-MNP) | PNIPAM- co-acrylic acid (p(NiPAAm-co-AAc)) coated with poly (ethyleneimine) (PEI) | Free radical polymerization | Gene delivery | Treatment with 20 mg/mL PEI-coated nanogels resulted in the maximum EGFP expression. After 24 h of transfection, EGFP expression was found for the first time, lasting up to 72 h. In hMSCs, self-assembled p(NiPAAm-co-dAAc) nanogels conjugated with the GFP gene were strongly expressed, suggesting they may be used for gene delivery. | [210] |
| Device Type | Model Drug | Polymer Formulation | Preparation Method | Applications | Results | References |
|---|---|---|---|---|---|---|
| Injectable hydrogel | Melatonin | PNIPAM/hyaluronic acid (HA) loaded chitosan-g-acrylic acid-coated PLGA (ACH/PLGA) | Single emulsion solvent evaporation | Cartilage tissue engineering | This system demonstrated excellent integration with genuine cartilage, and scanning electron microscopy pictures revealed an interconnected permeable structure. The hydrogels had exceptional MTT plus biocompatibility, and the live–dead assay demonstrated that WJMSCs could proliferate and survive. Overall, this injectable hydrogel proved to be an encouraging system for cartilage tissue engineering due to its increased mechanical properties, reduced syneresis, ability to sustain drug release, and high bioactivity. | [188] |
| Hydrogels | Mesenchymal stem cells (MSCs) | PNIPAM/chitosan | Freeze drying | Cartilage tissue engineering | The hydrogel solution’s residence duration inside the scaffold was determined to be 6 min for CSNI100 and 9 min for CSNI400. The swelling ratio of hybrid scaffolds was larger than that of chitosan-only scaffolds, and CSNI400 had a greater swelling ratio than CSNI100. In CSNI100 and CSNI400, the number of MSCs climbed by 58 and 108%. These findings imply that chitosan solid and PNIPAM hydrogels with a polymerization degree of 400 are found to be encouraging for cartilage tissue engineering. | [263] |
| Injectable hydrogel | Human dental pulp stem cells (hDPSCs) | PNIPAM-based copolymer/graphene oxide (GO)/chitosan (CS) crosslinked by beta glycerol phosphate (beta-GP) and genipin (GN) | Free radical copolymerization | Bone tissue engineering | Based on MTT, DAPI staining, and cell survival findings, the produced hydrogels provided a biomimetic ECM milieu for hDPSC proliferation and can be used as a novel BTE scaffold with good biocompatibility. The hydrogels ramped up the expression of osteogenic genes such as OCN and Runx 2, and activity of ALP and calcium deposition was enhanced. | [264] |
| Hydrogel | Oxacillin | PNIPAM/hydroxyapatite (HAp) | Electrochemical polymerization | Bone tissue engineering | The PNIPAM-HAp scaffolds were found to be very porous using SEM, and the HAp concentration appeared to govern the composite’s porosity. The scaffolds had original ingredients (no new chemical compounds were produced), and the ECP procedure did not affect the crystallinity of the HAp, according to XRD and FTIR analyses. Compared to the scaffolds with limited HAp content, the PNIPAM-HAp scaffolds with higher HAp content had a decreased oxacillin drug release rate. The oxacillin delivered from scaffolds maintained bacterial activity against P. aeruginosa and S. aureus for an extensive period. ECP seems to be a favorable methodology for producing PNIPAM-HAp scaffolds for BTE based on the data acquired from the above results. | [246] |
| Hydrogel | - | PNIPAM/cardiosphere derived cells (CDCs) | Free radical polymerization | Cardiac tissue engineering | Under static and dynamic stretching, the CDCs validated elastic modulus-dependent cardiac diversity, as revealed by gene and protein expressions of cardiac markers such as cTnI, Connexin43, CACNA1c, and MYH6. The expression of cardiac markers CACNA1c and MYH6 was considerably enhanced after 1 Hz frequency was applied to murine CDCs, indicating that they were driven to differentiate into cardiac lineage. In 40 kPa and 21 kPa hydrogels, the strain promoted CDC cardiac differentiation. These findings suggest that employing a 40 kPa hydrogel to transplant CDCs could result in optimum cardiac regeneration and differentiation. | [265] |
| Thermosensitive hydrogel | Brown adipose-derived stem cells (BASCs) | PNIPAM/single wall carbon nanotubes (SWCNTs) | Lyophilization | Cardiac tissue engineering | In vitro, SWCNTs with PNIPAM hydrogel demonstrated significantly more bioactivities to encapsulated cells than onefold PNIPAM. When utilized as a carrier, the technique improved seeded cell engraftment and survival in infarct myocardium, showing therapeutic efficacy following myocardial infarction. | [266] |
| Stimuli-responsive hydrogel | 5-amino salicylic acid (5-ASA) and ornidazole | PNIPAM/glycogen (Gly) (cl-Gly/PNIPAM) and crosslinked by ethylene glycol dimethacrylate (EGDMA) | Free radical polymerization | Intestinal tissue engineering | The produced hydrogel’s LCST was reported to be in the spectrum of 32.5–34 °C. The hydrogel was shown to be compatible with human mesenchymal stem cells (hMSCs). The medications were efficiently loaded into the hydrogel system, which released both medications in a controlled manner, with 96–97% of the medications remaining stable after two months. The created hydrogel could be used for colon-focused delivery because of the nature and component stability of the medications. | [267] |
| Thermoresponsive hydrogel | - | Polyacrylic acid (PAA)/norbornene-functionalized chitosan (CsNb) crosslinked by bistetrazine-PNIPAM (bisTz-PNIPAM) | reversible addition–fragmentation chain transfer (RAFT) polymerization | Intestinal tissue engineering | The Tz-Nb click reaction between bisTz-PNIPAM crosslinker and CsNb created chemical crosslinks that improved the hydrogel structure’s durability and produced pores in the hydrogel grid that allowed high drug load capacity and release. Because of the pH responsiveness of PAA, shrinkage behavior, and hydrogel porosity of PNIPAM, the hydrogel only gave a restricted medication release (8.5%) of 5-ASA at pH 2.2, but then it showed practically perfect delivery (92%) at pH 7.4 and 37 °C within 48 h. The hydrogels were nontoxic to human fibroblast cells and were biodegradable, indicating that they have a lot of potential as a medication delivery mechanism for the colon. | [268] |
| Hybrid hydrogel | Chlorhexidine diacetate | P-methacrylate arginine (M-Arg)/N-isopropylacrylamide (NIPAAm)/polyhexamethylene guanidinie phosphate (PHMG) [P(M-Arg/NIPAAm/PHMG)] crosslinked by N-N’methylene bisacrylamide. | Free radical copolymerization. | Wound dressing | Changing the monomer’s mass ratio controlled the hydrogels’ mechanical characteristics, swelling manner, and CHX release in vitro. The zwitterionic M-Arg monomer validated the hydrogel device’s resilience to protein adsorption. The hydrogels’ wound healing performance and safety were validated in an in vivo and cytotoxicity investigation. Ultimately, this research showed that hydrogels that possess long-term, anti-protein adsorption and antibacterial capabilities could effectively heal wounds. | [269] |
| Thermosensitive hydrogel | Superoxide dismutase (SOD) | PNIPAM/poly (γ-glutamic acid) | Free radical polymerization. | Wound dressing | The hydrogels had thermo-sensitivity at physiological temperature, and the phase transition temperature was 28.2 °C according to results from a differential scanning calorimeter and gelling action. SOD activity in vitro reached up to 85% after 10 h, which seemed beneficial in eradicating the superoxide anion. MTT experiments ensured that this hydrogel was biocompatible. The thermo-sensitive hydrogels had a longer-lasting SOD release, improved moisture retention, and higher water absorption. The device has significant application potential for wound repair and may effectively stimulate healing. | [270] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels 2022, 8, 454. https://doi.org/10.3390/gels8070454
Ansari MJ, Rajendran RR, Mohanto S, Agarwal U, Panda K, Dhotre K, Manne R, Deepak A, Zafar A, Yasir M, et al. Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels. 2022; 8(7):454. https://doi.org/10.3390/gels8070454
Chicago/Turabian StyleAnsari, Mohammad Javed, Rahul R. Rajendran, Sourav Mohanto, Unnati Agarwal, Kingshuk Panda, Kishore Dhotre, Ravi Manne, A. Deepak, Ameeduzzafar Zafar, Mohd Yasir, and et al. 2022. "Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art" Gels 8, no. 7: 454. https://doi.org/10.3390/gels8070454
APA StyleAnsari, M. J., Rajendran, R. R., Mohanto, S., Agarwal, U., Panda, K., Dhotre, K., Manne, R., Deepak, A., Zafar, A., Yasir, M., & Pramanik, S. (2022). Poly(N-isopropylacrylamide)-Based Hydrogels for Biomedical Applications: A Review of the State-of-the-Art. Gels, 8(7), 454. https://doi.org/10.3390/gels8070454