4.4. Methods
Zeta Potential and Dynamic Light Scattering. The Zeta potential (ζ) and hydrodynamic sizes of IL and IL/Water samples were determined by a Zetasizer Nano S (Malvern Instruments, Malvern, UK) equipped with a 4 mV He–Ne laser operating at a wavelength of 633 nm. Experiments were conducted at 25 °C, in disposable plastic cuvettes and using a non-invasive back-scattering angle of 173°. Prior to the measurements, all aqueous solutions were mildly shaken with a vortex system and filtered using sterile Millex PVDF filters with a pore diameter of 0.822 μm. Results are based on at least 3 parallel runs with 20 acquisitions each.
Density Measurements. The densities, ρ, were measured in the thermal range between 10 to 40 °C with a vibrating U-tube densimeter (DMA 4500, Anton Paar, Graz, Austria) and uncertainty of 5·10
−4 g·cm
−3 [
86]. The measurements were carried out 3 times and the obtained values were given as the mean with a maximum relative standard deviation of 0.5%.
Raman Spectroscopy. Raman measurements were carried out with a micro-Raman spectrometer LabRAM HR from Horiba Jobin with a laser at 532 nm for the IL/Water emulsions and 785 nm for both DOX loaded IL/Water emulsions and the Ionogels. The spectral resolution was around 1 cm−3 and a 10× microscope objective was used. The Raman spectra were studied by multivariate analysis using Eigenvectors developed by Horiba, the Raman spectra were pre-processed by SNV in order to normalize the spectra in the spectral region, to correct the noise remaining from the basic pre-processing done in the Raman spectrometer and its iCRaman software. The algorithm used for the input model is a Singular Value Decomposition (SVD) with full validation of the results.
Fourier-Transform Infrared Spectroscopy by Attenuated Total Reflectance (ATR-FTIR) was conducted using a Spectrometer Nicolet 6700, fitted with a source IR -Turbo fitted with a detector DTGS. The spectra of the Ionogels were obtained in a beamsplitter of KBr. The number of background scans was set to 34 and the tests were performed at ambient temperature with a spectral resolution of 4 cm−1. The Ionogels were deposited in the gold holder fitted with a humid chamber to prevent evaporation during the measurement. Background readings were subtracted from sample readings.
Rheological Experiments. The mechanical behavior was investigated using a Physica MCR 101 rheometer (Anton Paar, Graz, Austria) and a rotational Discovery Hybrid Rheometer DHR-2 (TA Instruments, New Castle, DE, USA) capable of controlling torques between 0.5 μN·m and 125 mN·m the former and between 2 nN·m and 200 mN·m for the latter. The viscosity values of the pure ionic liquid and the IL/Water obtained in this work from rotational and oscillatory tests present deviations lower than 8%.
The IL/Water emulsions flow tests were conducted with the Physica MCR 101 device at shear rates from 0.1 s−1 to 100 s−1 using a concentric cylinder geometry (cup: CC27/T200/SS with an inner diameter of 28.9 mm; and bob: B-CC27/P6 bob with outer diameter of 26.7 mm). The temperature was controlled by a C-PTD200 cylinder Peltier system. The declared uncertainty of the shear viscosity results obtained was below 4%. Oscillatory strain sweeps were also conducted for the IL/Water emulsions with the DHR-2 rheometer. A coaxial cylinder geometry together with a Peltier jacket was selected for the experiments. The geometry consisted of an external cup (diameter: 30.4 mm) and a bob (diameter: 28.0 mm, operating gap: 1.2 mm) in which the frequency was fixed at 0.05 Hz and the strain was increased from 1 to 1000%.
The Ionogels oscillatory analyses were conducted at 10 °C with the Physica MCR 101 rheometer operating with a plate-plate geometry (PP 25/S) and a gap of 0.1 mm. The temperature was controlled by a Peltier P-PTD 200, which was placed on the bottom plate. First, to identify the linear viscoelastic range (LVR), strain sweeps were performed at a constant pulsation of 10 rad/s and strains ranging from 0.01 to 1000%. Then, the storage modulus G’ and the loss modulus G” were determined over the linear strain range using a constant strain of 0.1% and covering frequencies from 0.1 to 200 rad/s. In addition, a temperature sweep of an oscillatory experiment was also carried out, in a temperature range of 10 to 40 °C at a constant frequency of 1Hz and a strain of 0.1 to 1%.
Prior to experiments, solutions were preheated at 60 °C for 30 min to homogenize samples and ensure proper geometry filling, as well as to avoid air bubbles. All rheological measurements were performed in triplicate to report reproducible data.
The
syringe test measurements were carried out with a geometry, PP25/S plate and a gap of 0.1 mm. The measurements involve 3 different stages [
42] in which an steady strain of 0.1% at 22 °C is first applied. Afterwards, the temperature is raised to 37 °C at a steady shear rate of 100 s
−1. The final step is to apply a strain of 0.1% at 37 °C. The reported data are given with deviations in the (complex) viscosities between repetitions less than 3%.
Cryo-Scanning Electron Microscopy (Cryo-SEM). The microstructures of the shark gelatin hydrogel (GE) and Ionogels at 5, 15, and 25% wt were probed in cryo mode. The samples were deposited on a gold 13 mm and frozen at −200 °C by means of liquid nitrogen; they were then immediately transferred under liquid nitrogen in a cryogen box, to the Baltec equipment (MODEL, MED-020). Afterwards, they were cut on the surface (with a thickness of 2 to 3 mm). The snapped samples were observed, and images were acquired with a scanning electron microscope (JEOL JSM-6700) at an acceleration voltage of 5 kV.
The morphology of the collapsed gelatin-based Ionogels after etching (where etching involves semi-drying) is then visualized which provides a coarse structure of the hydrogel [
87]. Moreover, a quantitative morphological analysis was performed with image J. The Interval Plot graph exhibits the average equivalent diameter or Aspect Ratio for the porous structure, the error bars represent 95% confidence intervals.
Atomic Force Microscope (AFM). The surface morphology of the GE and Ionogels was also investigated to proved further complementary structural information to the cryo-SEM analyses. For Atomic Force Microscopy, the instrument used is Veeco’s Multimode 8 Nanoscope V, Tapping Mode measurement mode. The Cantilever used was NCHV-A (Tip Roc < 10 nm, Cantilever Antimony (n) Doped Si, K = 40 N/m, Frequency= 339–388 KHz). The images shown have been obtained using the 11.9 μm scanner. Processing Software: Nanoscope v8.10, Nanoscope Analysis, Gwyddion 2.3. For the preparation of the measurements, an aliquot is taken and deposited on a silicon substrate previously fixed with adhesive on a steel support. Measurements have been made in two different areas, taking images with different fields of view/magnifications (10 × 10, 5 × 5, 1.5 × 1.5 μm2 and 600 × 600 nm2). The quantitative determination of the three-dimensional roughness parameters (Sa, Sq, Sz, Ssk and Sku) was achieved after proceeding with a “Cylinder and Tilt” correction to erase plane inclination and thus, produce micrographs with insignificant dependence on geometric deformations. The morphological analysis was conducted without the interpolation in the pixels with missing areas, although, the images were depicted with the interpolation “Data Restore” to enhance the micrograph quality.
Differential Scanning Calorimetry (DSC). The thermal properties of dry shark gelatin, the ionic liquid, the IL/Water emulsions as well as the GE and Ionogels were analysed by differential scanning calorimetry using a Q2000 (TA Instruments, New Castle, DE, USA). The cold source consists of a mechanical cooling unit capable of reaching cell temperatures down to −90 °C. DSC calibration was performed according to manufacturer specifications and temperature and enthalpy corrections were conducted using indium as standard (melting point, Tm = 156.61 °C). The measuring chamber was purged with a continuous dry-nitrogen flow rate of 50 mL/min. Prior to the tests, GE and Ionogels were sonicated at 60 °C in a low-power ultrasound bath for half an hour to ensure the homogeneity of the gelatin solutions. Approximately 15 mg of sample were hermetically sealed in Tzero aluminium capsules able to withstand pressures up to 0.3 MPa. The following three-step thermal protocol was used for all the materials. Samples were first cooled down to 1 °C and maintained at those conditions for 5 min to ensure a uniform initial temperature within the studied materials. Then, three heating and cooling runs in the temperature range between 1 and 80 °C with a scanning rate of 5 °C/min were programmed. Finally, samples were subject to cooling and heating ramps between −80 and 80 °C at 1 °C/min. In the case of the dry gelatin, the last heating scan was extended up to 200 °C. The weights of the DSC pans were obtained before and after the experiments to prove the absence of mass loss occurs. The experimental uncertainties in enthalpy and temperature determinations are estimated as 1.2 J/g and 0.3 K, respectively.
Modulated Differential Scanning Calorimetry (MDSC). IL/Water emulsions were also analysed by means of Modulated Differential Scanning Calorimeter (MDSC) in the temperature range between −80 and 80 °C. The scanning rate was 1 °C/min while the modulation period and amplitude were selected to 60 s and 0.159 °C, respectively, to ensure heat-iso conditions (i.e., in the heating process the temperature never decreases during the modulation).
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis). Samples were analysed using SDS-PAGE according to Laemmli [
88]. Briefly, gelatin samples were dissolved at 3 mg/mL in mixtures of ionic liquid: water with weight fractions from 0 to 33.3% of total solution weight. Each solution was diluted 1:2 in sample buffer containing 10.52% glycerol, 21% sodium dodecyl sulfate (SDS) (10%), 0.63% dithiothreitol (DTT), and 0.5-M Tris-HCl (pH 6.8) and heated for 5 min at 100 °C. Collagen from blue sharks extracted as previously described [
89] was also dissolved at 1 mg/mL and treated similarly. A 15 μL aliquot of each sample was applied to wells in a 7% SDS polyacrylamide separating gel (100 mm × 750 mm × 0.75 mm) and electrophoresis was performed at 15 mA by a Mini-Protean II cell (Bio-Rad, Hercules, CA, USA). Thereafter, 0.04% Coomassie Blue in 25% ethanol (
v/
v) and 8% acetic acid (v/v) was applied for 2 h to stain the gels. Repeated washes of destaining solution (25% ethanol (
v/
v), 8% acetic acid (
v/
v)) were performed to eliminate the surplus of stain. The assessment of the molecular weight was conducted by protein standards from Thermo-Scientific (Waltham, MA, USA) Pageruler Unstained High Range (250–60 kDa) and Pageruler Plus Prestained Protein Ladder (250–10 kDa).
SAXS-Small-Angle X-Ray Scattering WAXS-Wide Angle X-Ray Scattering. The nanostructure of both IL-water emulsions, as well as their corresponding gelatin hydrogels, was investigated by X-ray scattering experiments at different length scales by simultaneous SAXS-WAXS measurements. In addition, the Il-Water emulsions and the gelatin IL-hydrogels were subjected to thermal treatment by using a Linkam HFSX350 capillary stage to monitor the structural evolution during the thermal transitions.
Small-angle X-ray scattering (SAXS) and wide-angle X-ray scattering (WAXS) experiments were conducted in NCD-SWEET beamline at ALBA synchrotron (Cerdanyola del Vallès, Spain) as well as at DUBBLE at ESRF. The energy of the X rays was set to 12 keV which is equivalent to a wavelength of 1 Å; and a beam size of ca. 100 μm × 100 μm (FWHM) for Alba experiments and ca. 300 μm × 300 μm for DUBBLE measurements. The SAXS patterns were acquired using a Pilatus 1 M detector that is characterized by an active surface of 981 × 1043 pixels of 172 μm × 172 μm at a sample to detector distance of ca 2.5 m whereas the WAXS patterns were collected using either a Rayonix LX255-HS or a Pilatus 300 K-W that possess a sensitive area of either 85 × 255 mm
2 or 1472 × 195 pixels respectively and a corresponding pixel size of 40 μm× 40 μm or 172 μm × 172 μm. The scattering patterns of the SAXS (Silver behenate, AgBe) and WAXS (α-alumina, α-Al
2O
3) have been used to calibrate the scattering vector q, where q = 4πsinθ/λ and θ being half of the scattering angle and λ the beam wavelength. The collected 2D patterns were integrated using the Bubble [
90] free software applying the required corrections such as the background subtraction amended by X-ray transmission through the sample as well as the normalization upon the incident beam intensity to avoid beam fluctuations. The intensity of the scattering vector was calibrated using the scattering of water as a calibrant.
The IL-water emulsions and the gelatin hydrogel were inserted in a glass capillary of 1.5 mm (diameter) and closed prior to being introduced in the Linkam heating stage. The samples have been subjected to a thermal protocol involving a cooling step from 22 °C to −40 °C and the subsequent heating step to 40 °C at a rate of 5 °C/min.
The IL/Water emulsions experimental reduced data were adjusted to the weakly correlated nanoscale mass fractal aggregates model by an in-house program [
91,
92] that use the minimization routine of MINUIT [
93,
94] program created in CERN in which I(q) is adjusted to:
The parameters to be fitted are k, ξ, Rg, G and Dm, where k, with 0 < k < 6 depict the degree of correlations over a distance ξ, ξ is the correlation distance, G is the exponential (Guinier) prefactor, Rg is the radius of gyration, with 0 < Dm < 3 as the fractal dimension of the aggregate.
The Ionogels integrated SAXS profiles were fitted to a heterogeneous sphere with a fractal structure which is described with a radius of gyration 𝑅𝑔 associated with the fractal size and the fractal dimension
D that defines their compactness [
11,
95] with the previous program, and thus, the SAXS profile intensity (
I(
q)) was adjusted to:
with 𝜉
2 =
, 𝜉 being the correlation length.
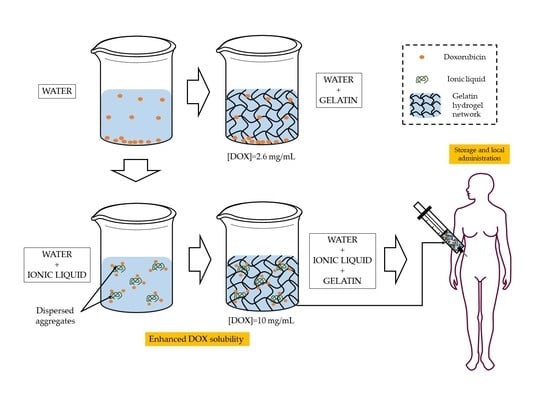
Solubility Test. The solubility of DOX was determined visually, by adding DOX to three different combinations of ionic fluid (FIL) and H2O mixtures. The ratios of FIL/ H2O were 0.1 g/0.65 g, and 0.25 g/0.5 g. Excess amounts of drugs were added to the mixtures to reach saturation concentration.
Firstly, 1 mg of DOX was added to each mixture and mixed at 3000 rpm with a Fisherbrand™ Vortex for 5 min followed by 5 min of an ultrasonic bath at room temperature (22 °C); these steps were repeated to reach concentrations of 2, 5, and finally 10 mg/mL of DOX.
In another experiment, the solubility of DOX was also tested in FIL without water. Excess amounts of drug were added to the mixtures to reach saturation concentration, but only Vortex mixing was needed this time.
Drug Release Experiments. 200 μL of each drug-loaded gelatin were located in the dialysis membrane (molecular weight cut-off: 12–14 kDa) and incubated in 8 mL of phosphate-buffered saline (PBS, pH 7.4) at 37 °C with continued mixing with a stirrer (300 rpm) in an IKA Plate (RCT digital). At different intervals of incubation, 1 mL of releasing medium was removed and new PBS was added to maintain the volume constant. The drug release profile was examined by recording UV-Vis spectra for 8 days. The drug-release concentration was measured in a JASCO V650 UV-Vis spectrophotometer at 403 and 483 nm for MTM and DOX, respectively, using PBS as a reference. The drugs released were tested in duplicates.


 ) Water, (
) Water, ( ) 1IL/Water, (
) 1IL/Water, ( ) 5IL/Water, (
) 5IL/Water, ( ) 10IL/Water, (
) 10IL/Water, ( ) 15IL/Water, (⯀) 25IL/Water and (
) 15IL/Water, (⯀) 25IL/Water and ( ) IL.
) IL.
 ) Water, (
) Water, ( ) 1IL/Water, (
) 1IL/Water, ( ) 5IL/Water, (
) 5IL/Water, ( ) 10IL/Water, (
) 10IL/Water, ( ) 15IL/Water, (⯀) 25IL/Water and (
) 15IL/Water, (⯀) 25IL/Water and ( ) IL.
) IL.
 ) Water, (
) Water, ( ) 1IL/Water, (
) 1IL/Water, ( ) 5IL/Water, (
) 5IL/Water, ( ) 10IL/Water, (
) 10IL/Water, ( ) 15IL/Water, (
) 15IL/Water, ( ) 25IL/Water and (
) 25IL/Water and ( ) IL.
) IL.
 ) Water, (
) Water, ( ) 1IL/Water, (
) 1IL/Water, ( ) 5IL/Water, (
) 5IL/Water, ( ) 10IL/Water, (
) 10IL/Water, ( ) 15IL/Water, (
) 15IL/Water, ( ) 25IL/Water and (
) 25IL/Water and ( ) IL.
) IL.












 ) GE, (
) GE, ( ) GE/1IL, (
) GE/1IL, ( ) GE/5IL, (
) GE/5IL, ( ) GE/10IL, (
) GE/10IL, ( ) GE/15IL and (🔴) GE/25IL.
) GE/15IL and (🔴) GE/25IL.
 ) GE, (
) GE, ( ) GE/1IL, (
) GE/1IL, ( ) GE/5IL, (
) GE/5IL, ( ) GE/10IL, (
) GE/10IL, ( ) GE/15IL and (🔴) GE/25IL.
) GE/15IL and (🔴) GE/25IL.