Alginate Hydrogels Reinforced by Dehydration under Stress—Application to a Soft Magnetic Actuator
Abstract
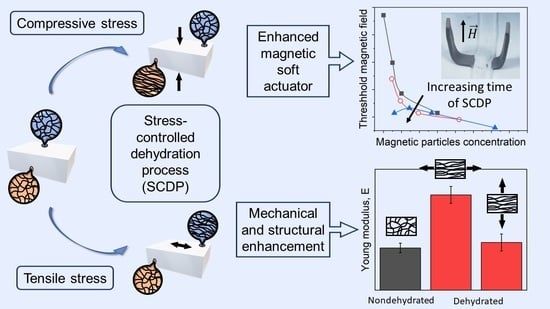
:1. Introduction
2. Results and Discussion
2.1. Macroscopic Appearance, Dehydration, and Re-Swelling of the Alginate Hydrogels
2.2. Mechanical Characterization of the Alginate Hydrogels
2.2.1. Mechanical Characterization under Tensile Stress
2.2.2. Mechanical Characterization under Oscillatory Shear Stress
2.3. Analysis of the Anisotropy in the Hydrogels
2.4. Role of SCDP in the Response of a Hydrogel-Based Magnetic Actuator
3. Conclusions
4. Materials and Methods
4.1. Synthesis of Nonmagnetic and Magnetic Hydrogels
4.2. Stress-Controlled Dehydration Processes (SCDP)
4.3. Mechanical Characterization of the Alginate Hydrogels
4.4. Scanning Electron Microscopy Imaging of the Hydrogel Microstructure
4.5. Analysis of the Actuation Behavior
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.Q.; Dong, S.J.; Xu, W.G.; Tu, S.C.; Yan, L.S.; Zhao, C.W.; Ding, J.X.; Chen, X.S. Antibacterial Hydrogels. Adv. Sci. 2018, 5, 1700527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.N.; Cong, Y.; Fu, J. Tissue adhesive hydrogel bioelectronics. J. Mat. Chem. B 2021, 9, 4423–4443. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, J. Bioinspired by cell membranes: Functional polymeric materials for biomedical applications. Mat. Chem. Front. 2020, 4, 750–774. [Google Scholar] [CrossRef]
- Raus, R.A.; Nawawi, W.M.F.W.; Nasaruddin, R.R. Alginate and alginate composites for biomedical applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Souri, H.; Banerjee, H.; Jusufi, A.; Radacsi, N.; Stokes, A.A.; Park, I.; Sitti, M.; Amjadi, M. Wearable and Stretchable Strain Sensors: Materials, Sensing Mechanisms, and Applications. Adv. Intell. Syst. 2020, 2, 2000039. [Google Scholar] [CrossRef]
- Wang, Z.W.; Cong, Y.; Fu, J. Stretchable and tough conductive hydrogels for flexible pressure and strain sensors. J. Mat. Chem. B 2020, 8, 3437–3459. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Nair, S.S.; Mishra, S.K.; Kumar, D. Recent progress in conductive polymeric materials for biomedical applications. Polym. Adv. Technol. 2019, 30, 2932–2953. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Orlova, T.; Chernozem, R.V.; Ivanova, A.A.; Bartasyte, A.; Mathur, S.; Surmeneva, M.A. Hybrid lead-free polymer-based nanocomposites with improved piezoelectric response for biomedical energy-harvesting applications: A review. Nano Energy 2019, 62, 475–506. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Amin, K.; An, Z.; Cai, Z.; Chen, H.; Chen, H.; Dong, Y.; Feng, X.; Fu, W.; Gu, J.; et al. Advanced functional polymer materials. Mat. Chem. Front. 2020, 4, 1803–1915. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, L.; Ma, P.X. Self-Healing Supramolecular Hydrogels for Tissue Engineering Applications. Macromol. Biosci. 2019, 19, 1800313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, X.X.; Lu, W.; Zhang, J.W.; Chen, T. Recent Progress in Biomimetic Anisotropic Hydrogel Actuators. Adv. Sci. 2019, 6, 1801584. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Zhao, X.H. Magnetic Soft Materials and Robots. Chem. Rev. 2022, 122, 5317–5364. [Google Scholar] [CrossRef]
- Taylor, D.L.; in het Panhuis, M. Self-Healing Hydrogels. Adv. Mater. 2016, 28, 9060–9093. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Uman, S.; Dhand, A.; Burdick, J.A. Recent advances in shear-thinning and self-healing hydrogels for biomedical applications. J. Appl. Polym. Sci. 2020, 137, 48668. [Google Scholar] [CrossRef]
- Kim, Y.; Yuk, H.; Zhao, R.; Chester, S.A.; Zhao, X. Printing ferromagnetic domains for untethered fast-transforming soft materials. Nature 2018, 558, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Goudu, S.R.; Yasa, I.C.; Hu, X.; Ceylan, H.; Hu, W.; Sitti, M. Biodegradable Untethered Magnetic Hydrogel Milli-Grippers. Adv. Funct. Mater. 2020, 30, 2004975. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Vazquez-Perez, F.J.; Gila-Vilchez, C.; Duran, J.D.G.; Zubarev, A.; de Cienfuegos, L.A.; Rodriguez-Arco, L.; Lopez-Lopez, M.T. Composite polymer hydrogels with high and reversible elongation under magnetic stimuli. Polymer 2021, 230, 124093. [Google Scholar] [CrossRef]
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020, 5, 20–43. [Google Scholar] [CrossRef]
- Du, H.S.; Liu, W.M.; Zhang, M.L.; Si, C.L.; Zhang, X.Y.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- Utech, S.; Prodanovic, R.; Mao, A.S.; Ostafe, R.; Mooney, D.J.; Weitz, D.A. Microfluidic Generation of Monodisperse, Structurally Homogeneous Alginate Microgels for Cell Encapsulation and 3D Cell Culture. Adv. Healthc. Mater. 2015, 4, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.; Mooney, D. Polymeric Tissue Adhesives. Chem. Rev. 2021, 121, 11336–11384. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. [Google Scholar] [CrossRef]
- Guo, B.L.; Ma, P.X. Conducting Polymers for Tissue Engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer Fiber Scaffolds for Bone and Cartilage Tissue Engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.S.; Zhou, F.; Liu, W.M.; Wang, Z.K. Mussel-inspired hydrogels: From design principles to promising applications. Chem. Soc. Rev. 2020, 49, 3605–3637. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Mredha, M.T.I.; Guo, Y.Z.; Nonoyama, T.; Nakajima, T.; Kurokawa, T.; Gong, J.P. A Facile Method to Fabricate Anisotropic Hydrogels with Perfectly Aligned Hierarchical Fibrous Structures. Adv. Mater. 2018, 30, 1704937. [Google Scholar] [CrossRef] [PubMed]
- Scionti, G.; Moral, M.; Toledano, M.; Osorio, R.; Durán, J.D.G.; Alaminos, M.; Campos, A.; López-López, M.T. Effect of the hydration on the biomechanical properties in a fibrin-agarose tissue-like model. J. Biomed. Mater. Res. A 2013, 102, 2573–2582. [Google Scholar] [CrossRef] [Green Version]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Poole, A.R.; Kojima, T.; Yasuda, T.; Mwale, F.; Kobayashi, M.; Laverty, S. Composition and Structure of Articular Cartilage. Clin. Orthop. Relat. Res. 2001, 391, S26–S33. [Google Scholar] [CrossRef]
- Fox, A.J.S.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sport. Health 2009, 1, 461–468. [Google Scholar]
- Weber, A.; Murray, J.M. Molecular control mechanisms in muscle contraction. Physiol. Rev. 1973, 53, 612–673. [Google Scholar] [CrossRef] [PubMed]
- Huxley, A. Muscular Contraction. Annu. Rev. Physiol. 1988, 50, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Madison, K.C. Barrier Function of the Skin: “La Raison d’Être” of the Epidermis. J. Invest. Dermatol. 2003, 121, 231–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proksch, E.; Brandner, J.M.; Jensen, J.-M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Prang, P.; Müller, R.; Eljaouhari, A.; Heckmann, K.; Kunz, W.; Weber, T.; Faber, C.; Vroemen, M.; Bogdahn, U.; Weidner, N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials 2006, 27, 3560–3569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Greenfield, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Aparicio, C.; de la Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef]
- McClendon, M.T.; Stupp, S.I. Tubular hydrogels of circumferentially aligned nanofibers to encapsulate and orient vascular cells. Biomaterials 2012, 33, 5713–5722. [Google Scholar] [CrossRef] [Green Version]
- Marelli, B.; Ghezzi, C.E.; James-Bhasin, M.; Nazhat, S.N. Fabrication of injectable, cellular, anisotropic collagen tissue equivalents with modular fibrillar densities. Biomaterials 2015, 37, 183–193. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Jeon, I. Biomimetic anisotropic hydrogels: Advanced fabrication strategies, extraordinary functionalities, and broad applications. Prog. Mater. Sci. 2022, 124, 100870. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of Anisotropic Hydrogels and Their Applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Gila-Vilchez, C.; Mañas-Torres, M.C.; Contreras-Montoya, R.; Alaminos, M.; Duran, J.D.G.; de Cienfuegos, L.Á.; Lopez-Lopez, M.T. Anisotropic magnetic hydrogels: Design, structure and mechanical properties. Philos. Trans. R. Soc. A-Math. Phys. Eng. Sci. 2019, 377, 20180217. [Google Scholar] [CrossRef]
- Lopez-Lopez, M.T.; Scionti, G.; Oliveira, A.C.; Duran, J.D.G.; Campos, A.; Alaminos, M.; Rodriguez, I.A. Generation and Characterization of Novel Magnetic Field-Responsive Biomaterials. PLoS ONE 2015, 10, e0133878. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.L.; Dai, C.F.; Wagner, D.; Daab, M.; Hong, W.; Breu, J.; Zheng, Q.; Wu, Z.L. Distributed Electric Field Induces Orientations of Nanosheets to Prepare Hydrogels with Elaborate Ordered Structures and Programmed Deformations. Adv. Mater. 2020, 32, 2005567. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, Y.; Coradin, T. Magnetically-oriented type I collagen-SiO2@Fe3O4 rods composite hydrogels tuning skin cell growth. Colloids Surf. B 2020, 185, 110597. [Google Scholar] [CrossRef] [PubMed]
- Mañas-Torres, M.C.; Gila-Vilchez, C.; Vazquez-Perez, F.J.; Kuzhir, P.; Momier, D.; Scimeca, J.-C.; Borderie, A.; Goracci, M.; Burel-Vandenbos, F.; Blanco-Elices, C.; et al. Injectable Magnetic-Responsive Short-Peptide Supramolecular Hydrogels: Ex Vivo and In Vivo Evaluation. ACS Appl. Mater. Interfaces 2021, 13, 49692–49704. [Google Scholar] [CrossRef]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Gladman, A.S.; Matsumoto, E.A.; Nuzzo, R.G.; Mahadevan, L.; Lewis, J.A. Biomimetic 4D printing. Nat. Mater. 2016, 15, 413–418. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J.; et al. Muscle-Inspired Highly Anisotropic, Strong, Ion-Conductive Hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Montoya, R.; Bonhome-Espinosa, A.B.; Orte, A.; Miguel, D.; Delgado-López, J.M.; Duran, J.D.G.; Cuerva, J.M.; Lopez-Lopez, M.T.; de Cienfuegos, L.Á. Iron nanoparticles-based supramolecular hydrogels to originate anisotropic hybrid materials with enhanced mechanical strength. Mat. Chem. Front. 2018, 2, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Zhang, T.; Li, N.; Cong, H.-P.; Yu, S.-H. Anisotropic and self-healing hydrogels with multi-responsive actuating capability. Nat. Commun. 2019, 10, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D bioprinting of cardiac micro-tissues mimicking native myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef] [PubMed]
- Gila-Vilchez, C.; Bonhome-Espinosa, A.B.; Kuzhir, P.; Zubarev, A.; Duran, J.D.G.; Lopez-Lopez, M.T. Rheology of magnetic alginate hydrogels. J. Rheol. 2018, 62, 1083–1096. [Google Scholar] [CrossRef] [Green Version]
- Barczak, M.; Borowski, P.; Gila-Vilchez, C.; Alaminos, M.; Gonzalez-Caballero, F.; Lopez-Lopez, M.T. Revealing importance of particles’ surface functionalization on the properties of magnetic alginate hydrogels. Carbohydr. Polym. 2020, 247, 116747. [Google Scholar] [CrossRef]
- Rubistein, M.; Colby, R.H. Polymer Physics; Oxford University: New York, NY, USA, 2003. [Google Scholar]
- Grosberg, A.; Khokhlov, A. Statistical Physics of Macromolecules; Springer: Berlin, Germany, 1994. [Google Scholar]
- Moghimi, E.; Jacob, A.R.; Koumakis, N.; Petekidis, G. Colloidal gels tuned by oscillatory shear. Soft Matter 2017, 13, 2371–2383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madge, D.S. The Control of Relative Humidity with Aqueous Solutions of Sodium Hydroxide. Entomol. Exp. Appl. 1961, 4, 143–147. [Google Scholar] [CrossRef]
- Draghi, L. Chapter Static and uniaxial characterization of polymer biomaterials. In Characterization of Polymeric Biomaterials; Tanzi, M.C., Farè, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 177–202. [Google Scholar]




| Stress Applied | Time | AH100-0 | AH50-50 | ||
|---|---|---|---|---|---|
| c (wt.%) | wl (wt.%) | c (wt.%) | wl (wt.%) | ||
| Compressive | 10 min | 4.72 ± 0.21 | 16 ± 4 | 4.41 ± 0.04 | 9.8 ± 0.9 |
| 20 min | 5.00 ± 0.16 | 21 ± 3 | 4.61 ± 0.07 | 13.8 ± 1.4 | |
| 30 min | 5.3 ± 0.3 | 26 ± 5 | 4.79 ± 0.07 | 17.1 ± 1.3 | |
| Tensile | 8 h | 4.67 ± 0.07 | 15.0 ± 1.4 | 4.87 ± 0.11 | 19 ± 2 |
| 14 h | 4.86 ± 0.12 | 18 ± 2 | 5.34 ± 0.10 | 26.1 ± 1.6 | |
| 24 h | 5.48 ± 0.21 | 28 ± 3 | 6.2 ± 0.3 | 37 ± 3 | |
| Fe-CC (% v/v) | c (wt.%) | |||
|---|---|---|---|---|
| FD | SD | FD | SD | |
| M5AH | 7.3 ± 1.4 | 8.0 ± 1.2 | 1.5 ± 0.3 | 1.7 ± 0.3 |
| M7.5AH | 9.7 ± 1.6 | 12.2 ± 2.5 | 1.33 ± 0.23 | 1.7 ± 0.4 |
| M10AH | 14.7 ± 1.2 | 18.4 ± 1.8 | 1.56 ± 0.15 | 2.03 ± 0.23 |
| M20AH | 26 ± 5 | 36 ± 6 | 1.4 ± 0.4 | 1.9 ± 0.6 |
| Sample | wt.% LVSA | wt.% MVSA | % v/v Fe-CC |
|---|---|---|---|
| C4.0AH100-0 | 4.00 | 0 | 0 |
| C4.0AH50-50 | 2.00 | 2.00 | 0 |
| C4.5AH100-0 | 4.50 | 0 | 0 |
| C4.5AH50-50 | 2.25 | 2.25 | 0 |
| C5.0AH100-0 | 5.00 | 0 | 0 |
| C5.0AH50-50 | 2.50 | 2.50 | 0 |
| C5.5AH100-0 | 5.50 | 0 | 0 |
| C5.5AH50-50 | 2.75 | 2.75 | 0 |
| M0AH | 0 | 1.00 | 0 |
| M5AH | 0 | 1.00 | 5.0 |
| M7.5AH | 0 | 1.00 | 7.5 |
| M10AH | 0 | 1.00 | 10.0 |
| M20AH | 0 | 1.00 | 20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leon-Cecilla, A.; Vazquez-Perez, F.J.; Gila-Vilchez, C.; Álvarez de Cienfuegos, L.; Lopez-Lopez, M.T. Alginate Hydrogels Reinforced by Dehydration under Stress—Application to a Soft Magnetic Actuator. Gels 2023, 9, 39. https://doi.org/10.3390/gels9010039
Leon-Cecilla A, Vazquez-Perez FJ, Gila-Vilchez C, Álvarez de Cienfuegos L, Lopez-Lopez MT. Alginate Hydrogels Reinforced by Dehydration under Stress—Application to a Soft Magnetic Actuator. Gels. 2023; 9(1):39. https://doi.org/10.3390/gels9010039
Chicago/Turabian StyleLeon-Cecilla, Alberto, Francisco J. Vazquez-Perez, Cristina Gila-Vilchez, Luis Álvarez de Cienfuegos, and Modesto T. Lopez-Lopez. 2023. "Alginate Hydrogels Reinforced by Dehydration under Stress—Application to a Soft Magnetic Actuator" Gels 9, no. 1: 39. https://doi.org/10.3390/gels9010039
APA StyleLeon-Cecilla, A., Vazquez-Perez, F. J., Gila-Vilchez, C., Álvarez de Cienfuegos, L., & Lopez-Lopez, M. T. (2023). Alginate Hydrogels Reinforced by Dehydration under Stress—Application to a Soft Magnetic Actuator. Gels, 9(1), 39. https://doi.org/10.3390/gels9010039