Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions
Abstract
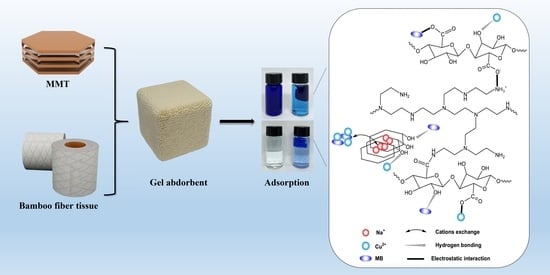
:1. Introduction
2. Results and Discussion
2.1. Characterization of the BMP Gel Adsorbent
2.1.1. FTIR Spectra
2.1.2. SEM
2.2. MB and Cu(II) Adsorption
2.2.1. Effect of Initial pH
2.2.2. Adsorption Kinetics and Isotherms
2.2.3. Effect of Interfering Ions
2.2.4. Adsorption Thermodynamics and Adsorbent Reusability
2.2.5. Adsorption Mechanism Analysis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Bamboo Nanocellulose (BCNF)
4.3. Preparation of MMTNS
4.4. Preparation of BCNF/MMTNS/PEI (BMP) Gel Adsorbent
4.5. Adsorbent Characterization
4.6. Adsorption Experiment
4.7. Reusability Experiment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Dai, L.; Yao, J.; Guo, T.; Hrynsphan, D.; Tatsiana, S.; Chen, J. Improvement of Alcaligenes sp.TB Performance by Fe-Pd/Multi-Walled Carbon Nanotubes: Enriched Denitrification Pathways and Accelerated Electron Transport. Bioresour. Technol. 2021, 327, 124785. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lu, K.; Hardison, A.K.; Liu, Z.; Xu, X.; Gao, D.; Gong, J.; Gardner, W.S. Membrane Inlet Mass Spectrometry Method (REOX/MIMS) to Measure 15N-Nitrate in Isotope-Enrichment Experiments. Ecol. Indic. 2021, 126, 107639. [Google Scholar] [CrossRef]
- Guan, Q.; Zeng, G.; Song, J.; Liu, C.; Wang, Z.; Wu, S. Ultrasonic Power Combined with Seed Materials for Recovery of Phosphorus from Swine Wastewater via Struvite Crystallization Process. J. Environ. Manag. 2021, 293, 112961. [Google Scholar] [CrossRef]
- Chen, F.; Ma, J.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Biodegradation Performance and Anti-Fouling Mechanism of an ICME/Electro-Biocarriers-MBR System in Livestock Wastewater (Antibiotic-Containing) Treatment. J. Hazard. Mater. 2022, 426, 128064. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Kang, S.; Qin, L.; Wang, W.; Zhang, T.; Song, S.; Komarneni, S. Self-Assembled Gels of Fe-Chitosan/Montmorillonite Nanosheets: Dye Degradation by the Synergistic Effect of Adsorption and Photo-Fenton Reaction. Chem. Eng. J. 2020, 379, 122322. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI -Containing Mg(OH)2 Nanowaste. Angew. Chem. Int. Ed. 2008, 47, 5619–5622. [Google Scholar] [CrossRef]
- Kabir, S.F.; Cueto, R.; Balamurugan, S.; Romeo, L.D.; Kuttruff, J.T.; Marx, B.D.; Negulescu, I.I. Removal of Acid Dyes from Textile Wastewaters Using Fish Scales by Absorption Process. Clean Technol. 2019, 1, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Bai, B.; Bai, F.; Li, X.; Nie, Q.; Jia, X.; Wu, H. The Remediation Efficiency of Heavy Metal Pollutants in Water by Industrial Red Mud Particle Waste. Environ. Technol. Innov. 2022, 28, 102944. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Gao, J.; Li, Z.; Wang, Z.; Chen, T.; Hu, G.; Zhao, Y.; Han, X. Facile Synthesis of Sustainable Tannin/Sodium Alginate Composite Hydrogel Beads for Efficient Removal of Methylene Blue. Gels 2022, 8, 486. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, J.; Kang, M.; Ma, C.; Li, H.; Qiu, Z. Hydroxyethyl Cellulose Hydrogel Modified with Tannic Acid as Methylene Blue Adsorbent. J. Appl. Polym. Sci. 2021, 138, 49880. [Google Scholar] [CrossRef]
- He, Y.; Jiang, D.B.; Chen, J.; Jiang, D.Y.; Zhang, Y.X. Synthesis of MnO2 Nanosheets on Montmorillonite for Oxidative Degradation and Adsorption of Methylene Blue. J. Colloid Interface Sci. 2018, 510, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Li, Q.; Li, X.; Su, Y.; Yue, Q.; Zhou, W.; Gao, B. Removal of Copper Ions from Aqueous Solutions by Adsorption onto Wheat Straw Cellulose-based Polymeric Composites. J. Appl. Polym. Sci. 2018, 135, 46680. [Google Scholar] [CrossRef]
- She, J.; Tian, C.; Wu, Y.; Li, X.; Luo, S.; Qing, Y.; Jiang, Z. Cellulose Nanofibrils Aerogel Cross-Linked by Poly(Vinyl Alcohol) and Acrylic Acid for Efficient and Recycled Adsorption with Heavy Metal Ions. J. Nanosci. Nanotechnol. 2018, 18, 4167–4175. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-X.; Jiang, H. Amino Modification of Biochar for Enhanced Adsorption of Copper Ions from Synthetic Wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef]
- Sun, X.-F.; Hao, Y.; Cao, Y.; Zeng, Q. Superadsorbent Hydrogel Based on Lignin and Montmorillonite for Cu(II) Ions Removal from Aqueous Solution. Int. J. Biol. Macromol. 2019, 127, 511–519. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Zhang, P.; Zhang, D.; Gao, F. Investigation on the Simultaneous Removal of Fluoride, Ammonia Nitrogen and Phosphate from Semiconductor Wastewater Using Chemical Precipitation. Chem. Eng. J. 2017, 307, 696–706. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Wang, Y.; Yang, Z.; Xu, T. The Reclamation of Aniline Wastewater and CO2 Capture Using Bipolar Membrane Electrodialysis. ACS Sustain. Chem. Eng. 2016, 4, 5743–5751. [Google Scholar] [CrossRef]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent Advancement of Coagulation–Flocculation and Its Application in Wastewater Treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Wang, M.; Sun, F.; Zeng, H.; Su, X.; Zhou, G.; Liu, H.; Xing, D. Modified Polyethersulfone Ultrafiltration Membrane for Enhanced Antifouling Capacity and Dye Catalytic Degradation Efficiency. Separations 2022, 9, 92. [Google Scholar] [CrossRef]
- Zeng, H.; Hao, H.; Wang, X.; Shao, Z. Chitosan-Based Composite Film Adsorbents Reinforced with Nanocellulose for Removal of Cu(II) Ion from Wastewater: Preparation, Characterization, and Adsorption Mechanism. Int. J. Biol. Macromol. 2022, 213, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Hong, H.-J.; Kim, S.M.; Ko, H.C.; Jeong, H.S. Mechanically Enhanced Graphene Oxide/Carboxymethyl Cellulose Nanofibril Composite Fiber as a Scalable Adsorbent for Heavy Metal Removal. Carbohydr. Polym. 2020, 240, 116348. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Ma, Q.; Jia, X.; Ma, P.-C. Preparation of Carbon Nanotubes/Graphene Hybrid Aerogel and Its Application for the Adsorption of Organic Compounds. Carbon 2017, 118, 765–771. [Google Scholar] [CrossRef]
- Azaman, S.A.H.; Afandi, A.; Hameed, B.H.; Din, A.T.M. Removal of Malachite Green from Aqueous Phase Using Coconut Shell Activated Carbon: Adsorption, Desorption, and Reusability Studies. J. Appl. Sci. Eng. 2018, 21, 317–330. [Google Scholar] [CrossRef]
- Yu, Z.; Wei, L.; Lu, L.; Shen, Y.; Zhang, Y.; Wang, J.; Tan, X. Structural Manipulation of 3D Graphene-Based Macrostructures for Water Purification. Gels 2022, 8, 622. [Google Scholar] [CrossRef]
- Zhu, L.; Zong, L.; Wu, X.; Li, M.; Wang, H.; You, J.; Li, C. Shapeable Fibrous Aerogels of Metal–Organic-Frameworks Templated with Nanocellulose for Rapid and Large-Capacity Adsorption. ACS Nano 2018, 12, 4462–4468. [Google Scholar] [CrossRef]
- Xiao, W.-D.; Xiao, L.-P.; Xiao, W.-Z.; Liu, K.; Zhang, Y.; Zhang, H.-Y.; Sun, R.-C. Cellulose-Based Bio-Adsorbent from TEMPO-Oxidized Natural Loofah for Effective Removal of Pb(II) and Methylene Blue. Int. J. Biol. Macromol. 2022, 218, 285–294. [Google Scholar] [CrossRef]
- Andrade Siqueira, T.C.; Zanette da Silva, I.; Rubio, A.J.; Bergamasco, R.; Gasparotto, F.; Aparecida de Souza Paccola, E.; Ueda Yamaguchi, N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Omer, A.M.; Ouyang, X. Adsorptive Removal of Cationic Methylene Blue Dye Using Carboxymethyl Cellulose/k-Carrageenan/Activated Montmorillonite Composite Beads: Isotherm and Kinetic Studies. Int. J. Biol. Macromol. 2018, 106, 823–833. [Google Scholar] [CrossRef]
- Gu, H.; Zhou, X.; Lyu, S.; Pan, D.; Dong, M.; Wu, S.; Ding, T.; Wei, X.; Seok, I.; Wei, S.; et al. Magnetic Nanocellulose-Magnetite Aerogel for Easy Oil Adsorption. J. Colloid Interface Sci. 2020, 560, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Voisin, H.; Bergström, L.; Liu, P.; Mathew, A. Nanocellulose-Based Materials for Water Purification. Nanomaterials 2017, 7, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, F.; Dinh, D.M.; Hsieh, Y.-L. Adsorption and Desorption of Cationic Malachite Green Dye on Cellulose Nanofibril Aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Hong, H.-J.; Ban, G.; Kim, H.S.; Jeong, H.S.; Park, M.S. Fabrication of Cylindrical 3D Cellulose Nanofibril(CNF) Aerogel for Continuous Removal of Copper(Cu2+) from Wastewater. Chemosphere 2021, 278, 130288. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, T.; Xiao, S.; He, H.; Yang, J.; Qin, Z. Preparation and Adsorption Performance of Functionalization Cellulose-Based Composite Aerogel. Int. J. Biol. Macromol. 2022, 211, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lei, Y.; Khan, M.A.; Wang, F.; Chu, Y.; Lei, W.; Xia, M.; Zhu, S. Adsorption Properties, Kinetics & Thermodynamics of Tetracycline on Carboxymethyl-Chitosan Reformed Montmorillonite. Int. J. Biol. Macromol. 2019, 124, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Jia, F.; Zhao, Y.; Wang, W.; Song, S.; Li, H.; Liu, C. Surface Wettability of Montmorillonite (0 0 1) Surface as Affected by Surface Charge and Exchangeable Cations: A Molecular Dynamic Study. Appl. Surf. Sci. 2018, 459, 148–154. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Chapter 2 Structures and Mineralogy of Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 19–86. ISBN 978-0-08-044183-2. [Google Scholar]
- Chen, T.; Yuan, Y.; Zhao, Y.; Rao, F.; Song, S. Preparation of Montmorillonite Nanosheets through Freezing/Thawing and Ultrasonic Exfoliation. Langmuir 2019, 35, 2368–2374. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Zhao, Y.; Bai, H.; Huang, M.; Zhang, T.; Song, S. High-Performance Two-Dimensional Montmorillonite Supported-Poly(Acrylamide-Co-Acrylic Acid) Hydrogel for Dye Removal. Environ. Pollut. 2020, 257, 113574. [Google Scholar] [CrossRef]
- Wang, W.; Ni, J.; Chen, L.; Ai, Z.; Zhao, Y.; Song, S. Synthesis of Carboxymethyl Cellulose-Chitosan-Montmorillonite Nanosheets Composite Hydrogel for Dye Effluent Remediation. Int. J. Biol. Macromol. 2020, 165, 1–10. [Google Scholar] [CrossRef]
- Melo, B.C.; Paulino, F.A.A.; Cardoso, V.A.; Pereira, A.G.B.; Fajardo, A.R.; Rodrigues, F.H.A. Cellulose Nanowhiskers Improve the Methylene Blue Adsorption Capacity of Chitosan-g-Poly(Acrylic Acid) Hydrogel. Carbohydr. Polym. 2018, 181, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and Highly Efficient Removal of Dye from Aqueous Solution Using Natural Locust Bean Gum Based Hydrogels as Adsorbent. Int. J. Biol. Macromol. 2020, 143, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of Cellulose-Based Hydrogel: A Review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Chen, Y.; Li, J.; Lu, J.; Ding, M.; Chen, Y. Synthesis and Properties of Poly(Vinyl Alcohol) Hydrogels with High Strength and Toughness. Polym. Test. 2022, 108, 107516. [Google Scholar] [CrossRef]
- Ren, H.; Gao, Z.; Wu, D.; Jiang, J.; Sun, Y.; Luo, C. Efficient Pb(II) Removal Using Sodium Alginate–Carboxymethyl Cellulose Gel Beads: Preparation, Characterization, and Adsorption Mechanism. Carbohydr. Polym. 2016, 137, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Ghriga, M.A.; Grassl, B.; Gareche, M.; Khodja, M.; Lebouachera, S.E.I.; Andreu, N.; Drouiche, N. Review of Recent Advances in Polyethylenimine Crosslinked Polymer Gels Used for Conformance Control Applications. Polym. Bull. 2019, 76, 6001–6029. [Google Scholar] [CrossRef]
- Rong, N.; Chen, C.; Ouyang, K.; Zhang, K.; Wang, X.; Xu, Z. Adsorption Characteristics of Directional Cellulose Nanofiber/Chitosan/Montmorillonite Aerogel as Adsorbent for Wastewater Treatment. Sep. Purif. Technol. 2021, 274, 119120. [Google Scholar] [CrossRef]
- Mokhtari, A.; Sabzi, M.; Azimi, H. 3D Porous Bioadsorbents Based on Chitosan/Alginate/Cellulose Nanofibers as Efficient and Recyclable Adsorbents of Anionic Dye. Carbohydr. Polym. 2021, 265, 118075. [Google Scholar] [CrossRef]
- Tong, D.S.; Wu, C.W.; Adebajo, M.O.; Jin, G.C.; Yu, W.H.; Ji, S.F.; Zhou, C.H. Adsorption of Methylene Blue from Aqueous Solution onto Porous Cellulose-Derived Carbon/Montmorillonite Nanocomposites. Appl. Clay Sci. 2018, 161, 256–264. [Google Scholar] [CrossRef]
- Wu, L.M.; Tong, D.S.; Zhao, L.Z.; Yu, W.H.; Zhou, C.H.; Wang, H. Fourier Transform Infrared Spectroscopy Analysis for Hydrothermal Transformation of Microcrystalline Cellulose on Montmorillonite. Appl. Clay Sci. 2014, 95, 74–82. [Google Scholar] [CrossRef]
- Xie, H.; Pan, Y.; Xiao, H.; Liu, H. Preparation and Characterization of Amphoteric Cellulose–Montmorillonite Composite Beads with a Controllable Porous Structure. J. Appl. Polym. Sci. 2019, 136, 47941. [Google Scholar] [CrossRef]
- Shehap, A.M.; Nasr, R.A.; Mahfouz, M.A.; Ismail, A.M. Preparation and Characterizations of High Doping Chitosan/MMT Nanocomposites Films for Removing Iron from Ground Water. J. Environ. Chem. Eng. 2021, 9, 104700. [Google Scholar] [CrossRef]
- Dao, T.B.T.; Ha, T.T.L.; Nguyen, T.D.; Le, H.N.; Ha-Thuc, C.N.; Nguyen, T.M.L.; Perre, P.; Nguyen, D.M. Effectiveness of Photocatalysis of MMT-Supported TiO2 and TiO2 Nanotubes for Rhodamine B Degradation. Chemosphere 2021, 280, 130802. [Google Scholar] [CrossRef] [PubMed]
- Long, L.-Y.; Li, F.-F.; Weng, Y.-X.; Wang, Y.-Z. Effects of Sodium Montmorillonite on the Preparation and Properties of Cellulose Aerogels. Polymers 2019, 11, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, F.S. Amides and Amines. In Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine; Springer US: Boston, MA, USA, 1971; pp. 165–172. ISBN 978-1-4684-1874-3. [Google Scholar]
- Singha, N.R.; Mahapatra, M.; Karmakar, M.; Dutta, A.; Mondal, H.; Chattopadhyay, P.K. Synthesis of Guar Gum-g-(Acrylic Acid-Co-Acrylamide-Co-3-Acrylamido Propanoic Acid) IPN via in Situ Attachment of Acrylamido Propanoic Acid for Analyzing Superadsorption Mechanism of Pb(II)/Cd(II)/Cu(II)/MB/MV. Polym. Chem. 2017, 8, 6750–6777. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Yi, H.; Chen, T.; Kang, S.; Li, H.; Song, S. Preparation and Characterization of Self-Assembly Hydrogels with Exfoliated Montmorillonite Nanosheets and Chitosan. Nanotechnology 2018, 29, 025605. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef]
- Mok, C.F.; Ching, Y.C.; Osman, N.A.A.; Muhamad, F.; Hai, N.D.; Choo, J.H.; Hassan, C.R. Adsorbents for Removal of Cationic Dye: Nanocellulose Reinforced Biopolymer Composites. J. Polym. Res. 2020, 27, 373. [Google Scholar] [CrossRef]
- Impert, O.; Katafias, A.; Kita, P.; Mills, A.; Pietkiewicz-Graczyk, A.; Wrzeszcz, G. Kinetics and Mechanism of a Fast Leuco-Methylene Blue Oxidation by Copper(Ii)–Halide Species in Acidic Aqueous Media. Dalton Trans. 2003, 3, 348–353. [Google Scholar] [CrossRef]
- Salama, A.; Abouzeid, R.E.; Awwad, N.S.; Ibrahium, H.A. New Sustainable Ionic Polysaccharides Fibers Assist Calcium Phosphate Mineralization as Efficient Adsorbents. Fibers Polym. 2021, 22, 1526–1534. [Google Scholar] [CrossRef]
- Thakur, S.; Pandey, S.; Arotiba, O.A. Development of a Sodium Alginate-Based Organic/Inorganic Superabsorbent Composite Hydrogel for Adsorption of Methylene Blue. Carbohydr. Polym. 2016, 153, 34–46. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Chemical Modification of Cellulosic Biopolymer and Its Use in Removal of Heavy Metal Ions from Wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Y.; Yi, H.; Chen, T.; Kang, S.; Zhang, T.; Rao, F.; Song, S. Pb(ΙΙ) Removal from Water Using Porous Hydrogel of Chitosan-2D Montmorillonite. Int. J. Biol. Macromol. 2019, 128, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A Novel Adsorbent TEMPO-Mediated Oxidized Cellulose Nanofibrils Modified with PEI: Preparation, Characterization, and Application for Cu(II) Removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Haerifar, M.; Azizian, S. Fractal-Like Adsorption Kinetics at the Solid/Solution Interface. J. Phys. Chem. C 2012, 116, 13111–13119. [Google Scholar] [CrossRef]
- Erdem, A.; Ngwabebhoh, F.A.; Yildiz, U. Novel MacroporousCryogels with Enhanced Adsorption Capability for the Removal of Cu(II) Ions from Aqueous Phase: Modelling, Kinetics and Recovery Studies. J. Environ. Chem. Eng. 2017, 5, 1269–1280. [Google Scholar] [CrossRef]
- Muntean, S.G.; Nistor, M.A.; Ianoș, R.; Păcurariu, C.; Căpraru, A.; Surdu, V.-A. Combustion Synthesis of Fe3O4/Ag/C Nanocomposite and Application for Dyes Removal from Multicomponent Systems. Appl. Surf. Sci. 2019, 481, 825–837. [Google Scholar] [CrossRef]
- Wang, Z.; Song, L.; Wang, Y.; Zhang, X.-F.; Yao, J. Construction of a Hybrid Graphene Oxide/Nanofibrillated Cellulose Aerogel Used for the Efficient Removal of Methylene Blue and Tetracycline. J. Phys. Chem. Solids 2021, 150, 109839. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene Blue Removal from Water Using the Hydrogel Beads of Poly(Vinyl Alcohol)-Sodium Alginate-Chitosan-Montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef]
- Shahnaz, T.; Padmanaban, V.C.; Narayanasamy, S. Surface Modification of Nanocellulose Using Polypyrrole for the Adsorptive Removal of Congo Red Dye and Chromium in Binary Mixture. Int. J. Biol. Macromol. 2020, 151, 322–332. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdem, A.; Yildiz, U. Synergistic Removal of Cu(II) and Nitrazine Yellow Dye Using an Eco-friendly Chitosan-montmorillonite Hydrogel: Optimization by Response Surface Methodology. J. Appl. Polym. Sci. 2016, 133, 43664. [Google Scholar] [CrossRef]
- Zhao, H.; Ouyang, X.-K.; Yang, L.-Y. Adsorption of Lead Ions from Aqueous Solutions by Porous Cellulose Nanofiber–Sodium Alginate Hydrogel Beads. J. Mol. Liq. 2021, 324, 115122. [Google Scholar] [CrossRef]
- Yang, Z.; Hou, J.; Miao, L.; Wu, J. Comparison of Adsorption Behavior Studies of Methylene Blue by Microalga Residue and Its Biochars Produced at Different Pyrolytic Temperatures. Environ. Sci. Pollut. Res. 2021, 28, 14028–14040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Omer, A.M.; Hu, Z.; Yang, L.-Y.; Ji, C.; Ouyang, X. Fabrication of Magnetic Bentonite/Carboxymethyl Chitosan/Sodium Alginate Hydrogel Beads for Cu (II) Adsorption. Int. J. Biol. Macromol. 2019, 135, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Pang, H.; Tan, Y.; Zhang, S.; Li, J. 3D Multi-Wall Perforated Nanocellulose-Based Polyethylenimine Aerogels for Ultrahigh Efficient and Reversible Removal of Cu(II) Ions from Water. Chem. Eng. J. 2019, 378, 122157. [Google Scholar] [CrossRef]
- Shariful, M.I.; Sepehr, T.; Mehrali, M.; Ang, B.C.; Amalina, M.A. Adsorption Capability of Heavy Metals by Chitosan/Poly(Ethylene Oxide)/Activated Carbon Electrospun Nanofibrous Membrane: Research Article. J. Appl. Polym. Sci. 2018, 135, 45851. [Google Scholar] [CrossRef]
- Esmaeili, Z.; Izadyar, S.; Hamzeh, Y.; Abdulkhani, A. Preparation and Characterization of Highly Porous Cellulose Nanofibrils/Chitosan Aerogel for Acid Blue 93 Adsorption: Kinetics, Isotherms, and Thermodynamics Analysis. J. Chem. Eng. Data 2021, 66, 1068–1080. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Huang, Y.; Zhu, H.; Jiang, L.; Teng, F.; Li, Y. Okara Nanocellulose Fabricated Using Combined Chemical and Mechanical Treatments: Structure and Properties. J. Mol. Liq. 2021, 335, 116231. [Google Scholar] [CrossRef]










| Model | Parameters | Adsorbate | |
|---|---|---|---|
| MB | Cu(II) | ||
| Pseudo-first-order | qe (mg/g) | 85.3679 | 54.593 |
| k1 (g/(mg·h)) | 6.0452 | 9.9634 | |
| R12 | 0.9024 | 0.7876 | |
| Reduced Chi-Sqr | 37.1686 | 17.3686 | |
| Pseudo-second-order | qe (mg/g) | 91.0366 | 56.5531 |
| k2 (g/(mg·h)) | 0.0923 | 0.2877 | |
| R22 | 0.989 | 0.9386 | |
| Reduced Chi-Sqr | 4.1878 | 5.0132 | |
| Fractal-like pseudo-second-order | qe (mg/g) | 94.8239 | 60.043 |
| k (g/(mg·h)) | 0.0548 | 0.0961 | |
| a | 0.7888 | 0.5773 | |
| R32 | 0.9983 | 0.978 | |
| Reduced Chi-Sqr | 0.7763 | 2.0980 | |
| Langmuir | qm (mg/g) | 173.3430 | 83.8704 |
| KL (L/mg) | 0.1935 | 0.0645 | |
| R42 | 0.8954 | 0.8824 | |
| RL (mg/L) | 0 < RL < 1 | 0 < RL < 1 | |
| Reduced Chi-Sqr | 684.1315 | 70.1838 | |
| Freundlich | n | 5.3604 | 5.3692 |
| KF (mg/g)/(L/mg) | 59.0696 | 27.2935 | |
| R52 | 0.9637 | 0.9326 | |
| Reduced Chi-Sqr | 235.3060 | 40.2597 | |
| Sips | qm (mg/g) | 361.8749 | 254.6286 |
| KS (L/mg) | 0.2042 | 0.1118 | |
| n | 3.8214 | 4.0920 | |
| R62 | 0.9683 | 0.9345 | |
| Reduced Chi-Sqr | 239.8241 | 44.7173 | |
| Adsorbent | Adsorbate | pH | T (°C) | Qmax (mg/g) | Ref. |
|---|---|---|---|---|---|
| Graphene oxide/CNF aerogel | MB | - | - | 111.2 | [70] |
| PVA/chitosan/MMT hydrogel | MB | 8 | 30 | 132.2 | [71] |
| Cellulose-derived carbon/MMT | MB | 8 | 25 | 138.1 | [50] |
| Sugarcane bagasse | MB | - | 45 | 9.41 | [53] |
| BMP gel adsorbent | MB | 10 | 25 | 361.9 | This work |
| CNF aerogel | Cu(II) | 6 | 29.85 | 30.0 | [14] |
| Cellulose/acrylonitrile/methacrylic acid | Cu(II) | 5.5 | 25 | 76.8 | [64] |
| TEMPO-oxidized CNF | Cu(II) | 5 | 30 | 52.3 | [66] |
| BMP gel adsorbent | Cu(II) | 5 | 25 | 254.6 | This work |
| Adsorbate | T (K) | Kc | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (Jmol/K) |
|---|---|---|---|---|---|
| MB | 298 | 1.9332 | −1.6331 | 14.8247 | 6.6408 |
| 303 | 2.1144 | −1.8863 | |||
| 308 | 2.3684 | −2.2079 | |||
| 313 | 2.5517 | −2.4377 | |||
| 318 | 2.8165 | −2.7377 | |||
| Cu(II) | 298 | 1.4213 | −0.8711 | 8.7197 | 3.8453 |
| 303 | 1.4331 | −0.9065 | |||
| 308 | 1.5145 | −1.0628 | |||
| 313 | 1.6681 | −1.3315 | |||
| 318 | 1.7390 | −1.4628 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, F.; Zhao, X.; Cao, J.; Liu, S.; Zhang, Y.; Yuan, Z.; Huang, X.; De Hoop, C.F.; Peng, X.; et al. Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels 2023, 9, 40. https://doi.org/10.3390/gels9010040
Zhang X, Li F, Zhao X, Cao J, Liu S, Zhang Y, Yuan Z, Huang X, De Hoop CF, Peng X, et al. Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels. 2023; 9(1):40. https://doi.org/10.3390/gels9010040
Chicago/Turabian StyleZhang, Xuelun, Feng Li, Xiyu Zhao, Jiwen Cao, Shuai Liu, You Zhang, Zihui Yuan, Xiaobo Huang, Cornelis F. De Hoop, Xiaopeng Peng, and et al. 2023. "Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions" Gels 9, no. 1: 40. https://doi.org/10.3390/gels9010040
APA StyleZhang, X., Li, F., Zhao, X., Cao, J., Liu, S., Zhang, Y., Yuan, Z., Huang, X., De Hoop, C. F., Peng, X., & Huang, X. (2023). Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels, 9(1), 40. https://doi.org/10.3390/gels9010040