Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation
Abstract
:1. Introduction
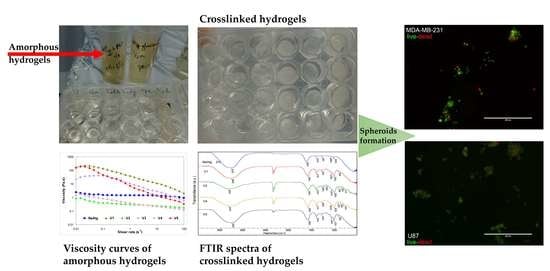
2. Results and Discussion
2.1. Structural and Rheological Investigation of Hydrogels
2.2. Evaluation of Spheroid Formation
3. Conclusions
4. Materials and Methods
4.1. Hydrogel Formulation
4.2. Structural, Morphological, and Reological Investigation of Hydrogels
4.3. Evaluation of Spheroid Formation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Markovitz-Bishitz, Y.; Tauber, Y.; Afrimzon, E.; Zurgil, N.; Sobolev, M.; Shafran, Y.; Deutsch, A.; Howitz, S.; Deutsch, M. A polymer microstructure array for the formation, culturing, and high throughput drug screening of breast cancer spheroids. Biomaterials 2010, 31, 8436–8444. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Advanced cell culture techniques for cancer drug discovery. Biology 2014, 3, 345–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-H.; Kim, T.-H. Recent Advances in Multicellular Tumor Spheroid Generation for Drug Screening. Biosensors 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Abdalla, A.M.E.; Xiao, L.; Yang, G. Biopolymer-Based Microcarriers for Three-Dimensional Cell Culture and Engineered Tissue Formation. Int. J. Mol. Sci. 2020, 21, 1895. [Google Scholar] [CrossRef] [Green Version]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising Applications of Tumor Spheroids and Organoids for Personalized Medicine. Cancers 2020, 12, 2727. [Google Scholar] [CrossRef]
- Turner, E.; Chen, L.; Foulke, J.G.; Gu, Z.; Tian, F. CRISPR/Cas9 Edited RAS & MEK Mutant Cells Acquire BRAF and MEK Inhibitor Resistance with MEK1 Q56P Restoring Sensitivity to MEK/BRAF Inhibitor Combo and KRAS G13D Gaining Sensitivity to Immunotherapy. Cancers 2022, 14, 5449. [Google Scholar] [CrossRef]
- Gong, C.L.; Cheng, J.; Su, J.; Zhao, H.; Liu, T.; Wen, X.; Zhao, P. Generation of multicellular tumor spheroids with microwell-based agarose scaffolds for drug testing. PLoS ONE 2015, 10, e0130348. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. Highthroughput 3D spheroid culture and drug testing using a hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Dongjin, L.; Chaenyung, C. Cell subtype-dependent formation of breast tumor spheroids and their variable responses to chemotherapeutics within microfluidics-generated 3D microgels with tunable mechanics. Mater. Sci. Eng. C 2020, 112, 110932. [Google Scholar] [CrossRef]
- Chen, C.Y.; Ke, C.J.; Yen, K.C.; Hsieh, H.C.; Sun, J.S.; Lin, F.H. 3D porous calcium-alginate scaffolds cell culture system improved human osteoblast cell clusters for cell therapy. Theranostics 2015, 5, 643–655. [Google Scholar] [CrossRef] [Green Version]
- Mazza, G.; Rombouts, K.; Hall, A.R.; Urbani, L.; Luong, T.V.; Al-Akkad, W.; Longato, L.; Brown, D.; Maghsoudlou, P.; Dhillon, A.P.; et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015, 5, 13079. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, C.; Wang, C.; Kuang, Y.; Li, Y.; Jiang, F.; Gong, A. Superflexible wood. ACS Appl. Mater. Inter. 2017, 9, 23520–23527. [Google Scholar] [CrossRef]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Vignesh, R.A.; Kumari, S.; Poddar, P.; Dhara, D.; Maiti, S. Poly(N-isopropylacrylamide)-Based Polymers as Additive for Rapid Generation of Spheroid via Hanging Drop Method. Macromol. Biosci. 2020, 20, 2000180. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Freed, L.E.; Vunjak-Novakovic, G.; Biron, R.J.; Eagles, D.B.; Lesnoy, D.C.; Barlow, S.K.; Langer, R. Biodegradable Polymer Scaffolds for Tissue Engineering. Biotechnology 1994, 12, 689–693. [Google Scholar] [CrossRef]
- Pham, H.M.; Zhang, Y.; Munguia-Lopez, J.G.; Tran, S.D. Egg White Alginate as a Novel Scaffold Biomaterial for 3D Salivary Cell Culturing. Biomimetics 2022, 7, 5. [Google Scholar] [CrossRef]
- Yunfeng, L.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [Green Version]
- Patil, P.S.; Mansouri, M.; Leipzig, N.D. Fluorinated Chitosan Microgels to Overcome Internal Oxygen Transport Deficiencies in Microtissue Culture Systems. Adv. Biosyst. 2020, 4, 1900250. [Google Scholar] [CrossRef] [PubMed]
- Flores-Torres, S.; Peza-Chavez, O.; Kuasne, H.; Munguia-Lopez, J.G.; Kort-Mascort, J.; Ferri, L.; Jiang, T.; Rajadurai, C.V.; Park, M.; Sangwan, V.; et al. Alginate–gelatin–Matrigel hydrogels enable the development and multigenerational passaging of patient-derived 3D bioprinted cancer spheroid models. Biofabrication 2021, 13, 025001. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Koshy, S.T.; Branco da Cunha, C.; Shin, J.W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ikram, M.; Subhan, F.; Kang, H.Y.; Lim, Y.; Lee, R.; Jin, S.; Yeong, Y.H.; Kwak, J.Y.; Na, Y.J.; et al. Alginate–marine collagen–agarose composite hydrogels as matrices for biomimetic 3D cell spheroid formation. RSC Adv. 2016, 6, 46952–46965. [Google Scholar] [CrossRef]
- Fischbach, C.; Kong, H.J.; Hsiong, S.X.; Evangelista, M.B.; Yuen, W.; Mooney, D.J. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc. Natl. Acad. Sci. USA 2009, 106, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Preda, P.; Enciu, A.-M.; Adiaconita, B.; Mihalache, I.; Craciun, G.; Boldeiu, A.; Aricov, L.; Romanitan, C.; Stan, D.; Marculescu, C.; et al. New Amorphous Hydrogels with Proliferative Properties as Potential Tools in Wound Healing. Gels 2022, 8, 604. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Gofman, I.V.; Chukhchin, D.G.; Malkov, A.V.; Mishanin, A.I.; Golovkin, A.S.; Pavlova, E.N.; Poshina, D.N.; Skorik, Y.A. Biophysical Characterization and Cytocompatibility of Cellulose Cryogels Reinforced with Chitin Nanowhiskers. Polymers 2022, 14, 2694. [Google Scholar] [CrossRef]
- Tran, T.T.; Hamid, Z.A.; Cheong, K.Y. A Review of Mechanical Properties of Scaffold in Tissue Engineering: Aloe Vera Composites. J. Phys. Conf. Ser, 2018; 1082, 012080. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter 2019, 15, 7852–7862. [Google Scholar] [CrossRef]
- Sánchez, M.; González-Burgos, E.; Iglesias, I.; Gómez-Serranillos, P.M. Pharmacological Update Properties of Aloe veraand its Major Active Constituents. Molecules 2020, 25, 1324. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Gandhi, S.; Chauhan, M.; Ma, S.; Das, S.; Ghosh, D.; Chandrasekharan, A.; Alam, B.; Parmar, A.S.; Sharma, A.; et al. Water-Templated, Polysaccharide-rich Bioartificial 3D Microarchitectures as Extra-Cellular Matrix Bioautomatons. ACS Appl. Mater. Interfaces 2020, 12, 20912–20921. [Google Scholar] [CrossRef]
- Kumar, S.; Tiku, A.B. Immunomodulatory potential of acemannan (polysaccharide from Aloe vera) against radiation induced mortality in Swiss albino mice. Food Agric. Immunol. 2016, 27, 72–86. [Google Scholar] [CrossRef]
- Jayakumar, R.; Nwe, N.; Tokura, S.; Tamura, H. Sulfated chitin and chitosan as novel biomaterials. Int. J. Biol. Macromol. 2007, 40, 175–181. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qian, J.; Zhang, Y.; Xu, W. Growth of MCF-7 breast cancer cells and efficacy of anti-angiogenic agents in a hydroxyethyl chitosan/glycidyl methacrylate hydrogel. Cancer Cell Int. 2017, 17, 55. [Google Scholar] [CrossRef] [Green Version]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Kievit, F.M.; Erickson, A.E.; Silber, J.R.; Ellenbogen, R.G.; Zhang, M. Culture on 3D Chitosan-Hyaluronic Acid Scaffolds Enhances Stem Cell Marker Expression and Drug Resistance in Human Glioblastoma Cancer Stem Cells. Adv. Healthc. Mater. 2016, 5, 3173–3181. [Google Scholar] [CrossRef]
- Kievit, F.M.; Stephen, J.F.; Leung, M.C.; Veiseh, O.; Park, J.O.; Disis, M.L.; Zhang, M. Chitosan–alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 2010, 31, 5903–5910. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudzadeh, A.; Mohammadpour, H. Tumor cell culture on collagen–chitosan scaffolds as three-dimensional tumor model: A suitable model for tumor studies. J. Food Drug Anal. 2016, 24, 620–626. [Google Scholar] [CrossRef] [Green Version]
- Erickson, A.E.; Lan Levengood, S.K.; Sun, J.; Chang, F.-C.; Zhang, M. Fabrication and Characterization of Chitosan-Hyaluronic Acid Scaffolds with Varying Stiffness for Glioblastoma Cell Culture. Adv. Healthc. Mater. 2018, 7, 1800295. [Google Scholar] [CrossRef]
- Morello, G.; Quarta, A.; Gaballo, A.; Moroni, L.; Gigli, G.; Polini, A.; Gervaso, F. A thermo-sensitive chitosan/pectin hydrogel for long-term tumor spheroid culture. Carbohydr. Polym. 2021, 274, 118633. [Google Scholar] [CrossRef]
- Mathews, E.H.; Stander, B.A.; Joubert, A.M.; Liebenberg, L. Tumor cell culture survival following glucose and glutamine deprivation at typical physiological concentrations. Nutrition 2014, 30, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Saleem, J.; Wang, L.M.; Chen, C.Y. Carbon-Based Nanomaterials for Cancer Therapy via Targeting Tumor Microenvironment. Adv. Healthc. Mater. 2018, 7, 1800525. [Google Scholar] [CrossRef] [PubMed]
- Madhusudana Rao, K.; Krishna Rao, K.S.V.; Sudhakar, P.; Chowdoji Rao, K.; Subha, M.C.S. Synthesis and characterization of biodegradable poly (vinyl caprolactam) grafted on to sodium alginate and its microgels for controlled release studies of an anticancer drug. J. Appl. Pharm. Sci. 2013, 3, 061–069. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between Alginate and Chitosan Biopolymers Characterized Using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef]
- Lim, Z.X.; Cheong, K.Y. Effects of drying temperature and ethanol concentration on bipolar switching characteristics of natural Aloe vera-based memory devices. Phys. Chem. Chem. Phys. 2015, 17, 26833. [Google Scholar] [CrossRef]
- Bialik-Was, K.; Raftopoulos, K.N.; Pielichowski, K. Alginate Hydrogels with Aloe vera: The Effects of Reaction Temperature on Morphology and Thermal Properties. Materials 2022, 15, 748. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V.; Dhiman, A.; Devi, M. Design of Aloe Vera-Alginate Gastroretentive Drug Delivery System to Improve the Pharmacotherapy. Polym. Plast. Technol. Eng. 2012, 51, 1303–1314. [Google Scholar] [CrossRef]
- Iovescu, A.; Stîngă, G.; Maxim, M.E.; Gosecka, M.; Basinska, T.; Slomkowski, S.; Angelescu, D.; Petrescu, S.; Stănică, N.; Băran, A.; et al. Chitosan-polyglycidol complexes to coating iron oxide particles for dye adsorption. Carbohydr. Polym. 2020, 246, 116571. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Badita, C.R.; Aranghel, D.; Burducea, C.; Mereuta, P. Characterization of sodium alginate based films. Rom. J. Phys. 2020, 65, 602. [Google Scholar]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef]
- Jabeen, S.; Maswal, M.; Chat, O.A.; Rather, G.M.; Dar, A.A. Rheological behavior and Ibuprofen delivery applications of pH responsive composite alginate hydrogels. Colloids Surf. B Biointerfaces 2016, 139, 211–218. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Lopez, F. Rheological Characterization of Hydrogels from Alginate-Based Nanodispersion. Polymers 2019, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Khan, M.; Rehman, T.; Ali, I.; Shah, L.A.; Khattak, N.S.; Khan, M.S. Synthesis and rheological survey of xanthan gum based terpolymeric hydrogels. Z. Phys. Chem. 2020, 235, 609–628. [Google Scholar] [CrossRef]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Promjantuek, W.; Heebkaew, N.; Rujanapun, N.; Noisa, P. Fabrication of 3D calcium-alginate scaffolds for human glioblastoma modeling and anticancer drug response evaluation. J. Cell. Physiol. 2019, 234, 20085–20097. [Google Scholar] [CrossRef]
- Dragoj, M.; Stojkovska, J.; Stanković, T.; Dinić, J.; Podolski-Renić, A.; Obradović, B.; Pešić, M. Development and Validation of a Long-Term 3D Glioblastoma Cell Culture in Alginate Microfibers as a Novel Bio-Mimicking Model System for Preclinical Drug Testing. Brain Sci. 2021, 11, 1025. [Google Scholar] [CrossRef]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.; Zhang, M. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-alginate scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef] [Green Version]
- Hamman, J.H. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [Green Version]
- Maan, A.A.; Nazir, A.; Khan, M.K.I.; Ahmad, T.; Zia, R.; Murid, M.; Abrar, M. The therapeutic properties and applications of aloe vera: A review. J. Herb. Med. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, X.; Liu, L.; Guo, M.; Huang, T.; Zhou, W.; Geng, F.; Cui, S.W.; Nie, S. Glucomannan from Aloe vera Gel Promotes Intestinal Stem Cell-Mediated Epithelial Regeneration via the Wnt/β-Catenin Pathway. J. Agric. Food Chem. 2021, 69, 10581–10591. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; de Leon-Rodriguez, A.; Kinsella, J.M. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh-Chau, N.L.; Kailei, X.; Zi, W.; Sean, B.; Robert, L.S.; Stephanie, J.F. Evaluation of the effect of 3D porous Chitosan-alginate scaffold stiffness on breast cancer proliferation and migration. J. Biomed. Mater. Res. Part A 2021, 109, 1990–2000. [Google Scholar] [CrossRef]
- Shen, Y.; Schmidt, B.U.S.; Kubitschke, H.; Morawetz, E.W.; Wolf, B.; Käs, J.A.; Losert, W. Detecting heterogeneity in and between breast cancer cell lines. Cancer Converg. 2020, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Enciu, A.-M.; Codrici, E.; Popescu, I.; Albulescu, L.; Dudau, M.; Costache, I.; Avram, A.; Tanase, C. Dextran-based polymers can be used as first choice to generate tumor spheroids in vitro. Ann. Oncol. 2022, 33, S1411. [Google Scholar] [CrossRef]
- Reiner, M.; Schoenfeld-Reiner, R. Viskosimetrische Untersuchungen an Lösungen hochmolekularer Naturstoffe. I. Mitteilung. Kautschuk in Toluol. Kolloid Z. 1933, 65, 44–62. [Google Scholar] [CrossRef]
- Rencber, S.C.; Cheaburu-Yılmaz, N.; Kose, F.A.; Karavana, S.Y.; Yılmaz, O. Preparation and Characterization of Alginate and Chitosan IPC based Gel Formulation for Mucosal Application. Cell Chem. Technol. 2019, 53, 655. [Google Scholar] [CrossRef]
- Williams, P.A.; Campbell, K.T.; Gharaviram, H.; Madrigal, J.L.; Silva, E.A. Alginate-chitosan hydrogels provide a sustained gradient of sphingosine-1-phosphate for therapeutic angiogenesis. Ann. Biomed. Eng. 2017, 45, 1003–1014. [Google Scholar] [CrossRef]







| Experimental Variants (Code) | Sodium Alginate (2%) (%) | Aloe Vera Powder (1%) (%) | Chitosan (1%) (%) | Glucose (1%) (%) | Additional Treatment |
|---|---|---|---|---|---|
| V1 | 70.6 | 29.4 | - | pH 5 | |
| V2 | 70.6 | 29.4 | - | pH 12 | |
| V3 | 70.4 | 29.3 | - | 0.3 | pH 5 |
| V4 | 70.4 | 29.3 | - | 0.3 | pH 12 |
| V5 | 50 | - | 50 | - | pH 5 |
| V6 | 41.4 | 17.2 | 41.4 | - | pH 5 |
| V7 | 41.4 | 17.2 | 41.4 | - | pH 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, P.; Enciu, A.-M.; Tanase, C.; Dudau, M.; Albulescu, L.; Maxim, M.-E.; Darie-Niță, R.N.; Brincoveanu, O.; Avram, M. Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation. Gels 2023, 9, 51. https://doi.org/10.3390/gels9010051
Preda P, Enciu A-M, Tanase C, Dudau M, Albulescu L, Maxim M-E, Darie-Niță RN, Brincoveanu O, Avram M. Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation. Gels. 2023; 9(1):51. https://doi.org/10.3390/gels9010051
Chicago/Turabian StylePreda, Petruța, Ana-Maria Enciu, Cristiana Tanase, Maria Dudau, Lucian Albulescu, Monica-Elisabeta Maxim, Raluca Nicoleta Darie-Niță, Oana Brincoveanu, and Marioara Avram. 2023. "Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation" Gels 9, no. 1: 51. https://doi.org/10.3390/gels9010051
APA StylePreda, P., Enciu, A. -M., Tanase, C., Dudau, M., Albulescu, L., Maxim, M. -E., Darie-Niță, R. N., Brincoveanu, O., & Avram, M. (2023). Assessing Polysaccharides/Aloe Vera–Based Hydrogels for Tumor Spheroid Formation. Gels, 9(1), 51. https://doi.org/10.3390/gels9010051