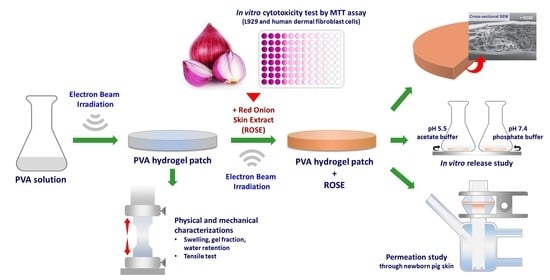
Electron Beam Irradiation Cross-Linked Hydrogel Patches Loaded with Red Onion Peel Extract for Transdermal Drug Delivery: Formulation, Characterization, Cytocompatibility, and Skin Permeation
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Yield of Thai ROSE
2.2. Physical Properties of Hydrogels
2.3. Mechanical Properties
2.4. Cytocompatibility of Hydrogel Patch
2.5. Morphology of ROSE-Coated Hydrogel Patch
2.6. Effect of ROSE Coating on Swelling and Mechanical Properties of Hydrogels
2.7. Decontamination of ROSE-Coated Hydrogel Patch
2.8. In Vitro Release Profile of ROSE from Hydrogel Patch
2.9. Permeation of ROSE from Hydrogel Patch through Newborn Pig Skin
2.10. FTIR Spectra of ROSE-Coated Hydrogel Patch
2.11. Cytocompatibility of ROSE at a Varied Concentration
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Plant Material and Extraction
4.3. Preparation of PVA Hydrogel Sheets by Electron Beam Irradiation
4.4. Characterization of Swelling, Gel Fraction, and Water Retention
4.5. Tensile Test
4.6. Coating of ROSE on Hydrogels
4.7. Morphology Analysis
4.8. In Vitro Release Profile of ROSE from the Hydrogel
4.9. Permeation Study of ROSE in Hydrogel through Newborn Pig Skin
4.10. FTIR Analysis of ROSE-Coated Hydrogel Patch
4.11. Cell Culture
4.12. In Vitro Cytotoxicity Test by MTT Assay
4.13. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- Hou, Y.; Jiang, N.; Sun, D.; Wang, Y.; Chen, X.; Zhu, S.; Zhang, L. A fast UV-curable PU-PAAm hydrogel with mechanical flexibility and self-adhesion for wound healing. RSC Adv. 2020, 10, 4907–4915. [Google Scholar] [CrossRef] [PubMed]
- Doersch, K.M.; Newell-Rogers, M.K. The impact of quercetin on wound healing relates to changes in αV and β1 integrin expression. Exp. Biol. Med. 2017, 242, 1424–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants and their natural components as future drugs for the treatment of burn wounds: An integrative review. Arch. Dermatol. Res. 2014, 306, 601–617. [Google Scholar] [CrossRef]
- Sidgwick, G.P.; McGeorge, D.; Bayat, A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch. Dermatol. Res. 2015, 307, 461–477. [Google Scholar] [CrossRef] [Green Version]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Ho, W.S.; Ying, S.Y.; Chan, P.C.; Chan, H.H. Use of onion extract, heparin, allantoin gel in prevention of scarring in chinese patients having laser removal of tattoos: A prospective randomized controlled trial. Dermatol. Surg. 2006, 32, 891–896. [Google Scholar] [CrossRef]
- Koc, E.; Arca, E.; Surucu, B.; Kurumlu, Z. An open, randomized, controlled, comparative study of the combined effect of intralesional triamcinolone acetonide and onion extract gel and intralesional triamcinolone acetonide alone in the treatment of hypertrophic scars and keloids. Dermatol. Surg. 2008, 34, 1507–1514. [Google Scholar] [CrossRef]
- Campanati, A.; Ceccarelli, G.; Brisigotti, V.; Molinelli, E.; Martina, E.; Talevi, D.; Marconi, B.; Giannoni, M.; Markantoni, V.; Gregoriou, S.; et al. Effects of in vivo application of an overnight patch containing Allium cepa, allantoin, and pentaglycan on hypertrophic scars and keloids: Clinical, videocapillaroscopic, and ultrasonographic study. Dermatol. Ther. 2021, 34, e14665. [Google Scholar] [CrossRef]
- Draelos, Z.D. The ability of onion extract gel to improve the cosmetic appearance of postsurgical scars. J. Cosmet. Dermatol. 2008, 7, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Vásquez, A.M.; Marín-Tello, C.L. Wound healing activity of Allium cepa L. bulbs in a second-degree burn wound model in Holtzman rats. Vitae 2021, 28, 1–12. [Google Scholar] [CrossRef]
- Marefati, N.; Ghorani, V.; Shakeri, F.; Boskabady, M.; Kianian, F.; Rezaee, R.; Boskabady, M.H. A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Marinozzi, M.; Baiocchi, C.; Beccari, T.; Calarco, P.; Ceccarini, M.R.; Chielli, M.; Orabona, C.; Orecchini, E.; Ortenzi, R.; et al. Bioadhesive Polymeric Films Based on Red Onion Skins Extract for Wound Treatment: An Innovative and Eco-Friendly Formulation. Molecules 2020, 25, 318. [Google Scholar] [CrossRef] [Green Version]
- Willital, G.H.; Heine, H. Efficacy of Contractubex gel in the treatment of fresh scars after thoracic surgery in children and adolescents. Int. J. Clin. Pharmacol. Res. 1994, 14, 193–202. [Google Scholar] [PubMed]
- Perez, O.A.; Viera, M.H.; Patel, J.K.; Konda, S.; Amini, S.; Huo, R.; Zell, D.; Tadicherla, S.; Berman, B. A comparative study evaluating the tolerability and efficacy of two topical therapies for the treatment of keloids and hypertrophic scars. J. Drugs Dermatol. 2010, 9, 514–518. [Google Scholar]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2019, 111, 134–151. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folzer, E.; Gonzalez, D.; Singh, R.; Derendorf, H. Comparison of skin permeability for three diclofenac topical formulations: An in vitro study. Pharmazie 2014, 69, 27–31. [Google Scholar]
- Poetschke, J.; Gauglitz, G.G. Hyperpigmented Scar. In Textbook on Scar Management: State of the Art Management and Emerging Technologies; Téot, L., Mustoe, T.A., Middelkoop, E., Gauglitz, G.G., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 505–507. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef] [PubMed]
- Phattanaphakdee, W.; Ditipaeng, C.; Uttayarat, P.; Thongnopkoon, T.; Athikomkulchai, S.; Chittasupho, C. Development and Validation of HPLC Method for Determination of Quercetin in Hydrogel Transdermal Patches Loaded with Red Onion Peel Extract. Trop. J. Nat. Prod. Res. 2022, 6, 1210–1214. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin Promotes Diabetic Wound Healing via Switching Macrophages from M1 to M2 Polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.W.; Cho, S.Y.; Lee, S.R.; Lee, K.S. Onion extract and quercetin induce matrix metalloproteinase-1 in vitro and in vivo. Int. J. Mol. Med. 2010, 25, 347–352. [Google Scholar]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Kianian, F.; Marefati, N.; Boskabady, M.; Ghasemi, S.Z.; Boskabady, M.H. Pharmacological Properties of Allium cepa, Preclinical and Clinical Evidences; A Review. Iran J. Pharm. Res. 2021, 20, 107–134. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Prawez, S. Hypoglycemic, Hypolipidemic, and Wound Healing Potential of Quercetin in Streptozotocin-Induced Diabetic Rats. Pharmacogn. Mag. 2017, 13, S633–S639. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Uttayarat, P.; Chiangnoon, R.; Eamsiri, J.; Senawongse, W. Processing and Characterization of Antibacterial Hydrogel Sheet Dressings Composed of Poly(vinyl alcohol) and Silk Fibroin for Wound Healing Application. Walailak J. Sci. Technol. 2018, 16, 349–359. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interactions Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Entezam, M.; Daneshian, H.; Nasirizadeh, N.; Khonakdar, H.A.; Jafari, S.H. Hybrid Hydrogels Based on Poly(vinyl alcohol) (PVA)/Agar/Poly(ethylene glycol) (PEG) Prepared by High Energy Electron Beam Irradiation: Investigation of Physico-Mechanical and Rheological Properties. Macromol. Mater. Eng. 2017, 302, 1600397. [Google Scholar] [CrossRef]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Baghaban Eslaminejad, M.; Tavakol, M.; Bagheri, F. Physicomechanical, rheological and in vitro cytocompatibility properties of the electron beam irradiated blend hydrogels of tyramine conjugated gum tragacanth and poly (vinyl alcohol). Mater. Sci. Eng. C 2020, 114, 111073. [Google Scholar] [CrossRef]
- Demeter, M.; Călina, I.; Vancea, C.; Şen, M.; Kaya, M.G.A.; Mănăilă, E.; Dumitru, M.; Meltzer, V. E-Beam Processing of Collagen-Poly(N-vinyl-2-pyrrolidone) Double-Network Superabsorbent Hydrogels: Structural and Rheological Investigations. Macromol. Res. 2019, 27, 255–267. [Google Scholar] [CrossRef]
- Călina, I.; Demeter, M.; Scărișoreanu, A.; Sătulu, V.; Mitu, B. One Step e-Beam Radiation Cross-Linking of Quaternary Hydrogels Dressings Based on Chitosan-Poly(Vinyl-Pyrrolidone)-Poly(Ethylene Glycol)-Poly(Acrylic Acid). Int. J. Mol. Sci. 2020, 21, 9236. [Google Scholar] [CrossRef]
- Haryanto; Kim, S.; Kim, J.H.; Kim, J.O.; Ku, S.; Cho, H.; Han, D.H.; Huh, P. Fabrication of poly(ethylene oxide) hydrogels for wound dressing application using E-beam. Macromol. Res. 2014, 22, 131–138. [Google Scholar] [CrossRef]
- Tabandeh, M.R.; Oryan, A.; Mohammadalipour, A. Polysaccharides of Aloe vera induce MMP-3 and TIMP-2 gene expression during the skin wound repair of rat. Int. J. Biol. Macromol. 2014, 65, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, M.; Dehshiri, S.; Vasheghani-Farahani, E. Electron beam irradiation crosslinked hydrogels based on tyramine conjugated gum tragacanth. Carbohydr. Polym. 2016, 152, 504–509. [Google Scholar] [CrossRef]
- Hafezi Moghaddam, R.; Dadfarnia, S.; Shabani, A.M.H.; Moghaddam, Z.H.; Tavakol, M. Electron beam irradiation synthesis of porous and non-porous pectin based hydrogels for a tetracycline drug delivery system. Mater. Sci. Eng. C 2019, 102, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Campanati, A.; Atzori, L.; Potenza, C.; Damiani, G.; Bianchi, L.; Corazza, M.; Tiberio, R.; Prignano, F.; Argenziano, G.; Fargnoli, M.C.; et al. Patient satisfaction with calcipotriol/betamethasone dipropionate cutaneous foam for the treatment of plaque psoriasis: The LION real-life multicenter prospective observational cohort study. Dermatol. Ther. 2021, 34, e15077. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Leawhiran, N.; Pavasant, P.; Soontornvipart, K.; Supaphol, P. Gamma irradiation synthesis and characterization of AgNP/gelatin/PVA hydrogels for antibacterial wound dressings. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Abd El-Mohdy, H.L.; Ghanem, S. Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J. Polym. Res. 2009, 16, 1–10. [Google Scholar] [CrossRef]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. Advanced SA/PVA-based hydrogel matrices with prolonged release of Aloe vera as promising wound dressings. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111667. [Google Scholar] [CrossRef]
- Soler, D.M.; Rodríguez, Y.; Correa, H.; Moreno, A.; Carrizales, L. Pilot scale-up and shelf stability of hydrogel wound dressings obtained by gamma radiation. Radiat. Phys. Chem. 2012, 81, 1249–1253. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Queen, D.; Gaylor, J.D.; Evans, J.H.; Courtney, J.M.; Reid, W.H. The preclinical evaluation of the water vapour transmission rate through burn wound dressings. Biomaterials 1987, 8, 367–371. [Google Scholar] [CrossRef]
- Yang, M.; Guo, W.; Liu, S.; Zhang, B.; Chen, Y.; Wang, Y. Highly stretchable gamma-irradiated poly (vinyl alcohol)/Tannic acid composite hydrogels with superior transparency and antibacterial activity. J. Polym. Res. 2021, 28, 412. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimton, P.; Ratphibun, P.; Tassaneesuwan, N.; Chiangnoon, R.; Uttayarat, P. Cytotoxicity Evaluation of Hydrogel Sheet Dressings Fabricated by Gamma Irradiation: Extract and Semi-Direct Contact Tests. Trends Sci. 2022, 19, 4583. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Dehghan Niri, T.; Heydari, M.; Hosseini, M.M. An improvement of adaptive cubic regularization method for unconstrained optimization problems. Int. J. Comput. Math. 2021, 98, 271–287. [Google Scholar] [CrossRef]
- Long, J.; Nand, A.V.; Bunt, C.; Seyfoddin, A. Controlled release of dexamethasone from poly(vinyl alcohol) hydrogel. Pharm. Dev. Technol. 2019, 24, 839–848. [Google Scholar] [CrossRef]
- Qindeel, M.; Ahmed, N.; Sabir, F.; Khan, S.; Ur-Rehman, A. Development of novel pH-sensitive nanoparticles loaded hydrogel for transdermal drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 629–641. [Google Scholar] [CrossRef]
- Calixto, G.; Yoshii, A.C.; Rocha e Silva, H.; Stringhetti Ferreira Cury, B.; Chorilli, M. Polyacrylic acid polymers hydrogels intended to topical drug delivery: Preparation and characterization. Pharm. Dev. Technol. 2015, 20, 490–496. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Sarunyoo, S. An Overview of skin penetration enhancers: Penetration enhancing activity, skin irritation potential and mechanism of action. Songklanakarin J. Sci. Technol. 2009, 31, 299–321. [Google Scholar]
- Butkeviciute, A.; Ramanauskiene, K.; Kurapkiene, V.; Janulis, V. Dermal Penetration Studies of Potential Phenolic Compounds Ex Vivo and Their Antioxidant Activity In Vitro. Plants 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Singh, S.S.J.; Rose, N.M. Phytochemical screening of onion skin (Allium cepa) dye extract. J. Pharmacogn. Phytochem. 2018, 7, 1414–1417. [Google Scholar]
- Ibezim-Ezeani, M. Diffusion Dynamics of Metal Ions Uptake at the CarboxylatedEpichlorohydrin Red Onion Skin Extract Resin—Aqueous Interface. Int. J. Eng. Res. Appl. 2017, 7, 46–52. [Google Scholar] [CrossRef]










| Water Content (%) | EDS (%) | Gel Fraction (%) | Rh (%) | ||
|---|---|---|---|---|---|
| 2 h | 24 h | ||||
| 10PVA hydrogel patches | 93.0 ± 0.6 | 1756.0 ± 156.1 | 89.6 ± 5.8 | 56.0 ± 8.9 | 9.6 ± 0.2 |
| Tensile Strength (kPa) | Elongation at Break (%) | Young’s Modulus (kPa) | |
|---|---|---|---|
| 10PVA hydrogel patches | 7.4 ± 1.7 | 72.3 ± 21.3 | 11.5 ± 1.4 |
| Samples | Batch 1 | Batch 2 | Batch 3 |
|---|---|---|---|
| Non-irradiated | 124 | 38 | 682 |
| Irradiated, 5 kGy | 0 | 0 | 0 |
| Irradiated, 10 kGy | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uttayarat, P.; Chiangnoon, R.; Thongnopkoon, T.; Noiruksa, K.; Trakanrungsie, J.; Phattanaphakdee, W.; Chittasupho, C.; Athikomkulchai, S. Electron Beam Irradiation Cross-Linked Hydrogel Patches Loaded with Red Onion Peel Extract for Transdermal Drug Delivery: Formulation, Characterization, Cytocompatibility, and Skin Permeation. Gels 2023, 9, 52. https://doi.org/10.3390/gels9010052
Uttayarat P, Chiangnoon R, Thongnopkoon T, Noiruksa K, Trakanrungsie J, Phattanaphakdee W, Chittasupho C, Athikomkulchai S. Electron Beam Irradiation Cross-Linked Hydrogel Patches Loaded with Red Onion Peel Extract for Transdermal Drug Delivery: Formulation, Characterization, Cytocompatibility, and Skin Permeation. Gels. 2023; 9(1):52. https://doi.org/10.3390/gels9010052
Chicago/Turabian StyleUttayarat, Pimpon, Rattanakorn Chiangnoon, Thanu Thongnopkoon, Kesinee Noiruksa, Jirachaya Trakanrungsie, Wattanaporn Phattanaphakdee, Chuda Chittasupho, and Sirivan Athikomkulchai. 2023. "Electron Beam Irradiation Cross-Linked Hydrogel Patches Loaded with Red Onion Peel Extract for Transdermal Drug Delivery: Formulation, Characterization, Cytocompatibility, and Skin Permeation" Gels 9, no. 1: 52. https://doi.org/10.3390/gels9010052
APA StyleUttayarat, P., Chiangnoon, R., Thongnopkoon, T., Noiruksa, K., Trakanrungsie, J., Phattanaphakdee, W., Chittasupho, C., & Athikomkulchai, S. (2023). Electron Beam Irradiation Cross-Linked Hydrogel Patches Loaded with Red Onion Peel Extract for Transdermal Drug Delivery: Formulation, Characterization, Cytocompatibility, and Skin Permeation. Gels, 9(1), 52. https://doi.org/10.3390/gels9010052