From Poly(glycerol itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications
Abstract
:1. Introduction
- Mild processing conditions (atmospheric pressure, room temperature) [6].
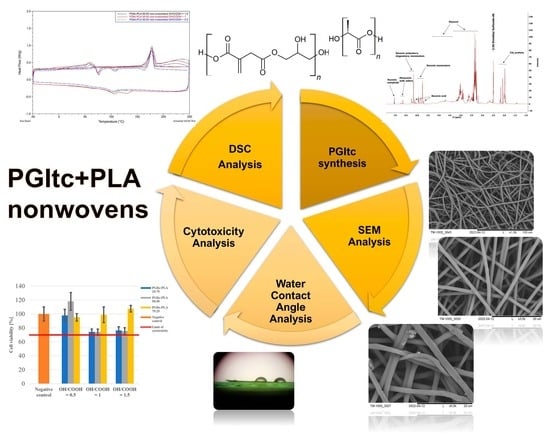
2. Results
2.1. PGItc Gel Characterization
- The band representing the stretching vibrations of the C=O carbonyl group (1709 cm−1);
- The stretching vibrations band of the C-O acyl group (1176 cm−1);
- The stretching vibrations band of the C-O alkoxy group (1036 cm−1).
2.2. SEM Images Analysis
2.3. Water Contact Angle Analysis
2.4. Cytotoxicity Analysis
2.5. Optical Profilometer Surface Imaging
2.6. Crosslinking and Leaching of the Nonwovens
2.7. DSC Analysis
3. Discussion
4. Conclusions
- The combination of poly(glycerol itaconate) and polylactide properties provides a non-cytotoxic material with cell viability above the cytotoxicity limit (>70%). PGItc can be used as fibers in subsequent investigations towards potential use in tissue engineering.
- The presence of unreacted monomers may have a negative impact on cell viability. In the future, conducting cytotoxicity studies of UV-crosslinked nonwovens will be beneficial to rearrange C=C bonds.
- The produced nonwovens were characterized as hydrophilic (contact angle < 90°). The higher proportion of poly(glycerol itaconate) and the lower OH/COOH ratio results in a product with better hydrophilic properties.
- Thermal crosslinking of PGItc + PLA nonwovens was successfully performed. Nonwoven leaching tests and DSC analysis of nonwoven samples confirmed it.
- The analysis of PGItc + PLA fibers made it possible to define the area of their potential application in tissue engineering. Based on the conducted studies, it was concluded that PGItc + PLA fibers could be used in the future as subcutaneous tissue fillers—for instance, for post-tumor defects, skin healing systems or as in vitro tumor models for cell-based compound screening (in drug discovery research).
- It appears to be interesting to test the potential use of PGItc in the form of hydrogels in medical applications.
- It is necessary to conduct further cytotoxicity studies (incubation time of 72 h) and mechanical tests.
5. Materials and Methods
5.1. PGItc Gels Synthesis Procedure
5.2. FTIR Analysis
5.3. Nuclear Magnetic Resonance (NMR) Spectroscopy
5.4. Electrospinning Procedure
5.5. Scanning Electron Microscopy
5.6. Water Contact Angle Measurements
5.7. Cytotoxicity Test
5.8. Optical Profilometer Surface Imaging
5.9. Crosslinking and Leaching of the Nonwovens
5.10. DSC Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for Tissue Engineering Applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Formhals, A. Methods and Apparatus for Spinning. U.S. Patent US2160962A, 30 May 1944. [Google Scholar]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of Electrospinning Technique for Biomedical Applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 013403. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Ding, M.; Shi, C.; Qiao, Y.; Wang, P.; Qiao, R.; Wang, X.; Zhong, J. Resorbable Polymer Electrospun Nanofibers: History, Shapes and Application for Tissue Engineering. Chin. Chem. Lett. 2020, 31, 617–625. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, F.; Sannino, A.; Peretti, G.M. The Biomaterialist’s Task: Scaffold Biomaterials and Fabrication Technologies. Joints 2013, 1, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, Y.; Chen, T. Techniques for Fabrication and Construction of Three-Dimensional Scaffolds for Tissue Engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, A.; Farkhani, S.M. Electrospinning and Electrospun Nanofibres. IET Nanobiotechnology 2014, 8, 83–92. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, X.; Zhang, G.; Wei, G.; Su, Z. Electrospinning Design of Functional Nanostructures for Biosensor Applications. J. Mater. Chem. B 2017, 5, 1699–1711. [Google Scholar] [CrossRef]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for Drug Delivery Applications: A Review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef]
- Feng, X.; Li, J.; Zhang, X.; Liu, T.; Ding, J.; Chen, X. Electrospun Polymer Micro/Nano Fi Bers as Pharmaceutical Repositories for Healthcare. J. Control. Release 2019, 302, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D. Review Article Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 2015, 140716. [Google Scholar] [CrossRef]
- Yari, A.; Yeganeh, H.; Bakhshi, H. Synthesis and Evaluation of Novel Absorptive and Antibacterial Polyurethane Membranes as Wound Dressing. J. Mater. Sci. Mater. Med. 2012, 23, 2187–2202. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Prabhakaran, M.P.; Ramakrishna, S. Stem Cell Differentiation to Epidermal Lineages on Electrospun Nanofibrous Substrates for Skin Tissue Engineering. Acta Biomater. 2011, 7, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; et al. Electrospun Gelatin/PCL and Collagen/PLCL Scaffolds for Vascular Tissue Engineering. Int. J. Nanomed. 2014, 9, 2335–2344. [Google Scholar]
- Islam, S.; Chin, B.; Andri, A.; Amalina, A.; Afifi, M. A Review on Fabrication of Nanofibers via Electrospinning and Their Applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-Responsive Bio-Based Polymeric Systems and Their Applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Rashid, T.U.; Gorga, R.E.; Krause, W.E. Mechanical Properties of Electrospun Fibers—A Critical Review. Adv. Eng. Mater. 2021, 23, 2100153. [Google Scholar] [CrossRef]
- De Oliveira, F.C.S.; Olvera, D.; Sawkins, M.J.; Cryan, S.A.; Kimmins, S.D.; Da Silva, T.E.; Kelly, D.J.; Duffy, G.P.; Kearney, C.; Heise, A. Direct UV-Triggered Thiol-Ene Cross-Linking of Electrospun Polyester Fibers from Unsaturated Poly(Macrolactone)s and Their Drug Loading by Solvent Swelling. Biomacromolecules 2017, 18, 4292–4298. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Sharifi, S.; Jahangiri, A. Electrospun Nanofibers as Versatile Platform in Antimicrobial Delivery: Current State and Perspectives. Pharm. Dev. Technol. 2019, 24, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of Silk Fibroin Nanofibers and Its Effect on the Adhesion and Spreading of Normal Human Keratinocytes and Fibroblasts In Vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Laurencin, C.; Kumbar, S.; Nukavarapu, S.; James, R.; Hogan, M. Recent Patents on Electrospun Biomedical Nanostructures: An Overview. Recent Pat. Biomed. Eng. 2010, 1, 68–78. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2021, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Duan, H.; Xue, J.; Liu, Y.; Feng, B.; Zhao, S.; Zhu, Y.; Liu, Y.; He, A.; Zhang, W.; et al. The Influence of Gelatin/PCL Ratio and 3-D Construct Shape of Electrospun Membranes on Cartilage Regeneration. Biomaterials 2014, 35, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Rayatpisheh, S.; Heath, D.E.; Shakouri, A.; Rujitanaroj, P.O.; Chew, S.Y.; Chan-Park, M.B. Combining Cell Sheet Technology and Electrospun Scaffolding for Engineered Tubular, Aligned, and Contractile Blood Vessels. Biomaterials 2014, 35, 2713–2719. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional Electrospun Nanofibrous Scaffolds for Biomedical Applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef]
- Ik, S.L.; Oh, H.K.; Meng, W.; Kang, I.K.; Ito, Y. Nanofabrication of Microbial Polyester by Electrospinning Promotes Cell Attachment. Macromol. Res. 2004, 12, 374–378. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Design Strategies of Tissue Engineering Scaffolds with Controlled Fiber Orientation. Tissue Eng. 2007, 13, 1845–1866. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Santo, V.E.; Gomes, M.E.; Mano, J.F.; Reis, R.L. From Nano-to Macro-Scale: Nanotechnology Approaches for Spatially Controlled Delivery of Bioactive Factors for Bone and Cartilage Engineering. Nanomedicine 2012, 7, 1045–1066. [Google Scholar] [CrossRef] [PubMed]
- Von Der Mark, K.; Park, J.; Bauer, S.; Schmuki, P. Nanoscale Engineering of Biomimetic Surfaces: Cues from the Extracellular Matrix. Cell Tissue Res. 2010, 339, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.X.; Ma, C.; Liang, Y.; Chen, F.M.; Liu, X. ECM-Mimicking Nanofibrous Matrix Coaxes Macrophages toward an Anti-Inflammatory Phenotype: Cellular Behaviors and Transcriptome Analysis. Appl. Mater. Today 2020, 18, 100508. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ma, J.; Chen, S.; Wu, S. Designing an Innovative Electrospinning Strategy to Generate PHBV Nanofiber Scaffolds with a Radially Oriented Fibrous Pattern. Nanomaterials 2023, 13, 1150. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.C.; Ferreira, I.; Borges, J.P. Electrospun Fibers in Composite Materials for Medical Applications. J. Compos. Biodegrad. Polym. 2013, 1, 56–65. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Yin, G.; Wang, H.; Dong, Z. Electrospun Polylactide/Silk Fibroin–Gelatin Composite Tubular Scaffolds for Small-Diameter Tissue Engineering Blood Vessels. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Yang, W.; Yang, F.; Wang, Y.; Both, S.K.; Jansen, J.A. In Vivo Bone Generation via the Endochondral Pathway on Three-Dimensional Electrospun Fibers. Acta Biomater. 2013, 9, 4505–4512. [Google Scholar] [CrossRef]
- Tallawi, M.; Dippold, D.; Rai, R.; D’Atri, D.; Roether, J.A.; Schubert, D.W.; Rosellini, E.; Engel, F.B.; Boccaccini, A.R. Novel PGS/PCL Electrospun Fiber Mats with Patterned Topographical Features for Cardiac Patch Applications. Mater. Sci. Eng. C 2016, 69, 569–576. [Google Scholar] [CrossRef]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The Antibacterial and Anti-Inflammatory Investigation of Lawsonia Inermis-Gelatin-Starch Nano-Fibrous Dressing in Burn Wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef]
- Ahmadi, T.; Monshi, A.; Mortazavi, V.; Fathi, M.H.; Sharifi, S.; Kharaziha, M.; Khazdooz, L.; Zarei, A.; Taghian Dehaghani, M. Fabrication and Characterization of Polycaprolactone Fumarate/Gelatin-Based Nanocomposite Incorporated with Silicon and Magnesium Co-Doped Fluorapatite Nanoparticles Using Electrospinning Method. Mater. Sci. Eng. C 2020, 106, 110172. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Multifunctional Poly(Glycolic Acid-Co-Propylene Fumarate) Electrospun Fibers Reinforced with Graphene Oxide and Hydroxyapatite Nanorods. J. Mater. Chem. B 2017, 5, 4084–4096. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Peng, H.; Wang, W.; Liu, T. Crystallization Behavior of Poly(ε-Caprolactone)/Layered Double Hydroxide Nanocomposites. J. Appl. Polym. Sci. 2010, 116, 2658–2667. [Google Scholar] [CrossRef]
- Romano, I.; Summa, M.; Heredia-Guerrero, J.A.; Spanó, R.; Ceseracciu, L.; Pignatelli, C.; Bertorelli, R.; Mele, E.; Athanassiou, A. Fumarate-Loaded Electrospun Nanofibers with Anti-Inflammatory Activity for Fast Recovery of Mild Skin Burns. Biomed. Mater. 2016, 11, 041001. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Huang, Y.C.; Leichner, J.; Nair, M.; Lin, W.C.; Li, C.Z. Peptide Modified Polymer Poly (Glycerol- Dodecanedioate Co-Fumarate) for Efficient Control of Motor Neuron Differentiation. Biomed. Mater. 2015, 10, 65013. [Google Scholar] [CrossRef] [PubMed]
- Kolankowski, K.; Miętus, M.; Ruśkowski, P.; Gadomska-Gajadhur, A. Optimisation of Glycerol and Itaconic Anhydride Polycondensation. Molecules 2022, 27, 4627. [Google Scholar] [CrossRef] [PubMed]
- Sriariyanun, M.; Heitz, J.H.; Yasurin, P.; Asavasanti, S.; Tantayotai, P. Itaconic Acid: A Promising and Sustainable Platform Chemical? Appl. Sci. Eng. Prog. 2019, 12, 75–82. [Google Scholar]
- Pagliaro, M.; Rossi, M. Glycerol: Properties and Production. In The Future of Glycerol, 2nd ed.; Clark, J.H., Kraus, G.A., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2010; ISBN 978-0-85404-124-4. [Google Scholar]
- Goyal, S.; Hernández, N.B.; Cochran, E.W. An Update on the Future Prospects of Glycerol Polymers. Polym. Int. 2021, 70, 911–917. [Google Scholar] [CrossRef]
- Sano, M.; Tanaka, T.; Ohara, H.; Aso, Y. Itaconic Acid Derivatives: Structure, Function, Biosynthesis, and Perspectives. Appl. Microbiol. Biotechnol. 2020, 104, 9041–9051. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Magalhães, A.I.; Soccol, C.R. Biobased Itaconic Acid Market and Research Trends-Is It Really a Promising Chemical? Chim. Oggi/Chem. Today 2018, 36, 56–58. [Google Scholar]
- Prabakaran, R.; Marie, J.M.; Xavier, A.J.M. Biobased Unsaturated Polyesters Containing Castor Oil-Derived Ricinoleic Acid and Itaconic Acid: Synthesis, In Vitro Antibacterial, and Cytocompatibility Studies. ACS Appl. Bio Mater. 2020, 3, 5708–5721. [Google Scholar] [CrossRef] [PubMed]
- Willke, T.; Vorlop, K.D. Biotechnological Production of Itaconic Acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, T.; Pardo, J.; Soo, K.; Peppas, N.A. Characterization of PH-Responsive Hydrogels of Poly(Itaconic Acid-g-Ethylene Glycol) Prepared by UV-Initiated Free Radical Polymerization as Biomaterials for Oral Delivery of Bioactive Agents. J. Biomed. Mater. Res. Part A 2010, 93, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Bujok, S.; Konefał, M.; Konefał, R.; Nevoralová, M.; Bednarz, S.; Mielczarek, K.; Beneš, H. Insight into the Aqueous Laponite® Nanodispersions for Self-Assembled Poly(Itaconic Acid) Nanocomposite Hydrogels: The Effect of Multivalent Phosphate Dispersants. J. Colloid Interface Sci. 2022, 610, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Murugan, R.; Wang, S.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(l-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Yan, Y.; Sencadas, V.; Jin, T.; Huang, X.; Chen, J.; Wei, D.; Jiang, Z. Tailoring the Wettability and Mechanical Properties of Electrospun Poly(L-Lactic Acid)-Poly(Glycerol Sebacate) Core-Shell Membranes for Biomedical Applications. J. Colloid Interface Sci. 2017, 508, 87–94. [Google Scholar] [CrossRef]
- Kolbuk, D.; Jeznach, O.; Wrzecionek, M.; Gadomska-Gajadhur, A. Poly(Glycerol Succinate) as an Eco-Friendly Component of PLLA and PLCL Fibres towards Medical Applications. Polymers 2020, 12, 1731. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Preparation of Cellulose Nanowhiskers and Their Reinforcing Effect in Polylactide. Macromol. Res. 2013, 21, 1218–1225. [Google Scholar] [CrossRef]
- Denis, P.; Wrzecionek, M.; Gadomska-Gajadhur, A.; Sajkiewicz, P. Poly(Glycerol Sebacate)-Poly(l-Lactide) Nonwovens. Towards Attractive Electrospun Material for Tissue Engineering. Polymers 2019, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Wrzecionek, M.; Bandzerewicz, A.; Dutkowska, E.; Dulnik, J.; Denis, P.; Gadomska-Gajadhur, A. Poly(Glycerol Citrate)-Polylactide Nonwovens toward Tissue Engineering Applications. Polym. Adv. Technol. 2021, 32, 3955–3966. [Google Scholar] [CrossRef]
- Kolankowski, K.; Gadomska-Gajadhur, A.; Wrzecionek, M.; Ruśkowski, P. Mathematically Described Model of Poly(Glycerol Maleate) Cross-linking Process Using Triethylenetetramine Addition. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/pat.5602 (accessed on 17 June 2022).
- Liu, Z.; Ramakrishna, S.; Liu, X. Electrospinning and Emerging Healthcare and Medicine Possibilities. APL Bioeng. 2020, 4, 030901. [Google Scholar] [CrossRef] [PubMed]
- Verreck, G.; Chun, I.; Rosenblatt, J.; Peeters, J.; Van Dijck, A.; Mensch, J.; Noppe, M.; Brewster, M.E. Incorporation of Drugs in an Amorphous State into Electrospun Nanofibers Composed of a Water-Insoluble, Nonbiodegradable Polymer. J. Control. Release 2003, 92, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Cavo, M.; Serio, F.; Kale, N.R.; D’Amone, E.; Gigli, G.; Del Mercato, L.L. Electrospun Nanofibers in Cancer Research: From Engineering of: From Vitro 3D Cancer Models to Therapy. Biomater. Sci. 2020, 8, 4887–4905. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.; Sánchez-Leija, R.J.; Gross, R.A.; Linhardt, R.J. Review on the Impact of Polyols on the Properties of Bio-Based Polyesters. Polymers 2020, 12, 2969. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Berlioz, S.; Chailan, J.F.; Panaitescu, D.M. Morphology and Thermal Properties of PLA-Cellulose Nanofibers Composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Hernández-López, M.; Correa-Pacheco, Z.N.; Bautista-Baños, S.; Zavaleta-Avejar, L.; Benítez-Jiménez, J.J.; Sabino-Gutiérrez, M.A.; Ortega-Gudiño, P. Bio-Based Composite Fibers from Pine Essential Oil and PLA/PBAT Polymer Blend. Morphological, Physicochemical, Thermal and Mechanical Characterization. Mater. Chem. Phys. 2019, 234, 345–353. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L. Calorimetric Analysis of the Multiple Melting Behavior of Poly(L-Lactic Acid). J. Appl. Polym. Sci. 2006, 100, 3145–3151. [Google Scholar] [CrossRef]










| PGItc:PLA Ratio | OH/COOH Ratio | ||
|---|---|---|---|
| 0.5 | 1 | 1.5 | |
| 25:75 | 3.08 ± 0.27 | 1.80 ± 0.08 | 3.28 ± 0.17 |
| 50:50 | 2.51 ± 0.24 | 2.00 ± 0.35 | 2.26 ± 0.05 |
| 75:25 | 1.74 ± 0.12 | 1.55 ± 0.24 | 1.26 ± 0.14 |
| Reactant | OH/COOH Ratio | ||
|---|---|---|---|
| 0.5 | 1 | 1.5 | |
| Itaconic Anhydride (IAn) | 23.55 g (0.210 mol) | 19.38 g (0.173 mol) | 16.47 g (0.147 mol) |
| Glycerol (G) | 6.45 g (0.070 mol) | 10.62 g (0.115 mol) | 13.53 g (0.147 mol) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miętus, M.; Kolankowski, K.; Gołofit, T.; Denis, P.; Bandzerewicz, A.; Spychalski, M.; Mąkosa-Szczygieł, M.; Pilarek, M.; Wierzchowski, K.; Gadomska-Gajadhur, A. From Poly(glycerol itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications. Gels 2023, 9, 788. https://doi.org/10.3390/gels9100788
Miętus M, Kolankowski K, Gołofit T, Denis P, Bandzerewicz A, Spychalski M, Mąkosa-Szczygieł M, Pilarek M, Wierzchowski K, Gadomska-Gajadhur A. From Poly(glycerol itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications. Gels. 2023; 9(10):788. https://doi.org/10.3390/gels9100788
Chicago/Turabian StyleMiętus, Magdalena, Krzysztof Kolankowski, Tomasz Gołofit, Piotr Denis, Aleksandra Bandzerewicz, Maciej Spychalski, Marcin Mąkosa-Szczygieł, Maciej Pilarek, Kamil Wierzchowski, and Agnieszka Gadomska-Gajadhur. 2023. "From Poly(glycerol itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications" Gels 9, no. 10: 788. https://doi.org/10.3390/gels9100788
APA StyleMiętus, M., Kolankowski, K., Gołofit, T., Denis, P., Bandzerewicz, A., Spychalski, M., Mąkosa-Szczygieł, M., Pilarek, M., Wierzchowski, K., & Gadomska-Gajadhur, A. (2023). From Poly(glycerol itaconate) Gels to Novel Nonwoven Materials for Biomedical Applications. Gels, 9(10), 788. https://doi.org/10.3390/gels9100788