Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior
Abstract
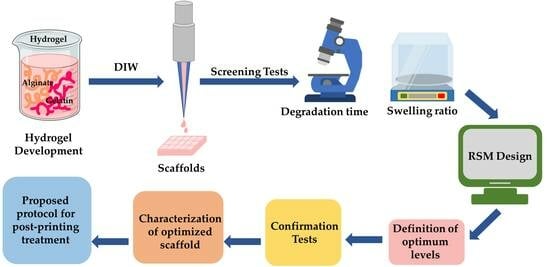
:1. Introduction
2. Results and Discussion
2.1. Scaffolds Development
2.2. Screening Tests Characterizations Results
2.2.1. Degradation Behavior
2.2.2. Swelling Ratio
2.3. Design of Experiments
2.3.1. Model Summary and Regression Analysis for Response 1: Degradation Time and Response 2: Swelling Ratio
2.3.2. Parameter Optimization by Response Surface Methodology for Scaffold’s Post-Printing Treatment
2.3.3. Model Validation
2.4. Confirmation Tests
2.4.1. RSM
2.4.2. Characterization Results for the Confirmation Tests
Degradation Behavior
- FT-IR Spectroscopy
- Shape retention
- Morphology
Swelling Behavior
- Swelling Ratio
3. Conclusions
4. Materials and Methods
4.1. Design of Experiments
4.2. Screening Tests of DoE’s Selected Levels
4.3. Materials
4.4. Hydrogel Synthesis
4.5. Direct Ink Writing of the Scaffolds
4.6. Crosslinking Process
4.7. UV-Exposure
4.8. Degradation Test
4.9. Swelling Test
4.10. FT-IR Spectroscopy
4.11. Scanning Electron Microscopy
4.12. Flow Diagram
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saadi, M.A.S.R.; Maguire, A.; Pottackal, N.T.; Thakur, M.S.H.; Ikram, M.M.; Hart, A.J.; Ajayan, P.M.; Rahman, M.M. Direct Ink Writing: A 3D Printing Technology for Diverse Materials. Adv. Mater. 2022, 34, e2108855. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Fu, J.; Lin, H.; He, Y. 3D Bioprinting: A Novel Avenue for Manufacturing Tissues and Organs. Engineering 2019, 5, 777–794. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Li Tan, M.; Viktorova, J.; Bennett, C.W.; Connal, L.A. Extrusion 3D Printing of Polymeric Materials with Advanced Properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R.; et al. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Saini, G.; Segaran, N.; Mayer, J.L.; Saini, A.; Albadawi, H.; Oklu, R. Applications of 3D Bioprinting in Tissue Engineering and Regenerative Medicine. J. Clin. Med. 2021, 10, 4966. [Google Scholar] [CrossRef]
- Yavvari, P.; Laporte, A.; Elomaa, L.; Schraufstetter, F.; Pacharzina, I.; Daberkow, A.D.; Hoppensack, A.; Weinhart, M. 3D-Cultured Vascular-Like Networks Enable Validation of Vascular Disruption Properties of Drugs In Vitro. Front. Bioeng. Biotechnol. 2022, 10, 888492. [Google Scholar] [CrossRef]
- Di Giuseppe, M.; Law, N.; Webb, B.; Macrae, R.A.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate–gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Lagopati, N.; Pavlatou, E.A. Advanced Applications of Biomaterials Based on Alginic Acid. Am. J. Biomed. Sci. 2020, 9, 47–53. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Erezuma, I.; Lukin, I.; Hernáez-Moya, R.; Orive, G. Composite alginate–gelatin hydrogels incorporating PRGF enhance human dental pulp cell adhesion, chemotaxis and proliferation. Int. J. Pharm. 2022, 617, 121631. [Google Scholar] [CrossRef]
- Lagopati, N.; Pippa, N.; Gatou, M.-A.; Papadopoulou-Fermeli, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Marine-Originated Materials and Their Potential Use in Biomedicine. Appl. Sci. 2023, 13, 9172. [Google Scholar] [CrossRef]
- Tomić, S.L.; Babić Radić, M.M.; Vuković, J.S.; Filipović, V.V.; Nikodinovic-Runic, J.; Vukomanović, M. Alginate-Based Hydrogels and Scaffolds for Biomedical Applications. Mar. Drugs 2023, 21, 177. [Google Scholar] [CrossRef]
- Ketabat, F.; Maris, T.; Duan, X.; Yazdanpanah, Z.; Kelly, M.E.; Badea, I.; Chen, X. Optimization of 3D Printing and in Vitro Characterization of Alginate/Gelatin Lattice and Angular Scaffolds for Potential Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1161804. [Google Scholar] [CrossRef]
- Wierzbicka, A.; Bartniak, M.; Rosińska, K.; Bociąga, D. Optimization of the preparation process stages of the bioink compositions based on sodium alginate and gelatin to improve the viability of biological material contained in hydrogel 3D printouts. Eng. Biomater. 2022, 165, 7–16. [Google Scholar] [CrossRef]
- Chawla, D.; Kaur, T.; Joshi, A.; Singh, N. 3D Bioprinted Alginate–gelatin Based Scaffolds for Soft Tissue Engineering. Int. J. Biol. Macromol. 2020, 144, 560–567. [Google Scholar] [CrossRef]
- Mondal, A.; Gebeyehu, A.; Miranda, M.; Bahadur, D.; Patel, N.; Ramakrishnan, S.; Rishi, A.K.; Singh, M. Characterization and printability of Sodium alginate -Gelatin hydrogel for bioprinting NSCLC co-culture. Sci. Rep. 2019, 9, 19914. [Google Scholar] [CrossRef]
- Sarker, M.; Izadifar, M.; Schreyer, D.; Chen, X. Influence of ionic crosslinkers (Ca2+/Ba2+/Zn2+) on the mechanical and biological properties of 3D Bioplotted Hydrogel Scaffolds. J. Biomater. Sci. Polym. Ed. 2018, 29, 1126–1154. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xiong, L.; Hu, Y.; Li, W.; Chen, Z.; Liu, K.; Zhang, X. Three-Dimensional Printing Alginate/Gelatin Scaffolds as Dermal Substitutes for Skin Tissue Engineering. Polym. Eng. Sci. 2018, 58, 1782–1790. [Google Scholar] [CrossRef]
- Amr, M.; Dykes, I.; Counts, M.; Kernan, J.; Mallah, A.; Mendenhall, J.; Van Wie, B.; Abu-Lail, N.; Gozen, B.A. 3D Printed, Mechanically Tunable, Composite Sodium Alginate, Gelatin and Gum Arabic (SA-GEL-GA) Scaffolds. Bioprinting 2021, 22, e00133. [Google Scholar] [CrossRef]
- Naghieh, S.; Karamooz-Ravari, M.R.; Sarker, M.D.; Karki, E.; Chen, X. Influence of crosslinking on the mechanical behavior of 3D printed alginate scaffolds: Experimental and numerical approaches. J. Mech. Behav. Biomed. Mater. 2018, 80, 111–118. [Google Scholar] [CrossRef]
- Bahrami, N.; Farzin, A.; Bayat, F.; Goodarzi, A.; Salehi, M.; Goodarzi, A.; Salehi, M.; Karimi, R.; Mohamadnia, A.; Parhiz, A.; et al. Optimization of 3D Alginate Scaffold Properties with Interconnected Porosity Using Freeze-drying Method for Cartilage Tissue Engineering Application. Arch. Neurosci. 2019, 6, e85122. [Google Scholar] [CrossRef]
- Sonaye, S.Y.; Ertugral, E.G.; Kothapalli, C.R.; Sikder, P. Extrusion 3D (Bio)Printing of Alginate–gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering. Materials 2022, 15, 7945. [Google Scholar] [CrossRef] [PubMed]
- Kaliampakou, C.; Lagopati, N.; Charitidis, C.A. Direct Ink Writing of Alginate–Gelatin Hydrogel: An Optimization of Ink Property Design and Printing Process Efficacy. Appl. Sci. 2023, 13, 8261. [Google Scholar] [CrossRef]
- Gupta, P.; Nayak, K.K. Optimization of Keratin/Alginate Scaffold Using RSM and Its Characterization for Tissue Engineering. Int. J. Biol. Macromol. 2016, 85, 141–149. [Google Scholar] [CrossRef]
- Pepelnjak, T.; Stojšić, J.; Sevšek, L.; Movrin, D.; Milutinović, M. Influence of Process Parameters on the Characteristics of Additively Manufactured Parts Made from Advanced Biopolymers. Polymers 2023, 15, 716. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Li, C.; Wu, Y.; Li, Q.; Liao, J. Hybrid Cellulose Nanocrystal/Alginate/Gelatin Scaffold with Improved Mechanical Properties and Guided Wound Healing. RSC Adv. 2019, 9, 22966–22979. [Google Scholar] [CrossRef]
- Aljohani, W.; Ullah, M.W.; Li, W.; Shi, L.; Zhang, X.; Yang, G. Three-Dimensional Printing of Alginate–gelatin-Agar Scaffolds Using Free-Form Motor Assisted Microsyringe Extrusion System. J. Polym. Res. 2018, 25, 62. [Google Scholar] [CrossRef]
- Li, Y.; Jia, H.; Cheng, Q.; Pan, F.; Jiang, Z. Sodium alginate–gelatin polyelectrolyte complex membranes with both high water vapor permeance and high permselectivity. J. Membr. Sci. 2011, 375, 304–312. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012019. [Google Scholar] [CrossRef]
- Gupta, N.V.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar]
- Talaei, A.; O’Connell, C.D.; Sayyar, S.; Maher, M.; Yue, Z.; Choong, P.F.; Wallace, G.G. Optimizing the composition of gelatin methacryloyl and hyaluronic acid methacryloyl hydrogels to maximize mechanical and transport properties using response surface methodology. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 526–537. [Google Scholar] [CrossRef]
- Coşkun, S.; Akbulut, S.O.; Sarıkaya, B.; Çakmak, S.; Gümüşderelioğlu, M. Formulation of Chitosan and Chitosan-nanoHAp Bioinks and Investigation of Printability with Optimized Bioprinting Parameters. Int. J. Biol. Macromol. 2022, 222, 1453–1464. [Google Scholar] [CrossRef] [PubMed]
- El Magri, A.; El Mabrouk, K.; Vaudreuil, S.; Ebn Touhami, M. Experimental Investigation and Optimization of Printing Parameters of 3D Printed Polyphenylene Sulfide through Response Surface Methodology. J. Appl. Polym. Sci. 2021, 138, 49625. [Google Scholar] [CrossRef]
- Vates, U.K.; Kanu, N.J.; Gupta, E.; Singh, G.K.; Daniel, N.A.; Sharma, B.P. Optimization of FDM 3D Printing Process Parameters on ABS Based Bone Hammer Using RSM Technique. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1206, 012001. [Google Scholar] [CrossRef]
- Dhote, V.; Vernerey, F.J. Mathematical model of the role of degradation on matrix development in hydrogel scaffold. Biomech. Model. Mechanobiol. 2014, 13, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Lagopati, N.; Kotsinas, A.; Veroutis, D.; Evangelou, K.; Papaspyropoulos, A.; Arfanis, M.; Falaras, P.; Kitsiou, P.V.; Pateras, I.; Bergonzini, A.; et al. Biological Effect of Silver-modified Nanostructured Titanium Dioxide in Cancer. Cancer Genom. Proteom. 2021, 18 (Suppl. 3), 425–439. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Carranza, T.; Zalba-Balda, M.; Baraibar, M.J.B.; de la Caba, K.; Guerrero, P. Effect of sterilization processes on alginate/gelatin inks for three-dimensional printing. Int. J. Bioprint. 2022, 9, 645. [Google Scholar] [CrossRef]
- Yen, S.; Sokolenko, S.; Manocha, B.; Blondeel, E.J.; Aucoin, M.G.; Patras, A.; Daynouri-Pancino, F.; Sasges, M. Treating cell culture media with UV irradiation against adventitious agents: Minimal impact on CHO performance. Biotechnol. Prog. 2014, 30, 1190–1195. [Google Scholar] [CrossRef]
- Katifelis, H.; Nikou, M.-P.; Mukha, I.; Vityuk, N.; Lagopati, N.; Piperi, C.; Farooqi, A.A.; Pippa, N.; Efstathopoulos, E.P.; Gazouli, M. Ag/Au Bimetallic Nanoparticles Trigger Different Cell Death Pathways and Affect Damage Associated Molecular Pattern Release in Human Cell Lines. Cancers 2022, 14, 1546. [Google Scholar] [CrossRef]
- Papadopoulou-Fermeli, N.; Lagopati, N.; Pippa, N.; Sakellis, E.; Boukos, N.; Gorgoulis, V.G.; Gazouli, M.; Pavlatou, E.A. Composite Nanoarchitectonics of Photoactivated Titania-Based Materials with Anticancer Properties. Pharmaceutics 2023, 15, 135. [Google Scholar] [CrossRef] [PubMed]
- Celina, M.; Ottesen, D.K.; Gillen, K.T.; Clough, R.L. FTIR emission spectroscopy applied to polymer degradation. Polym. Degrad. Stab. 1997, 58, 15–31. [Google Scholar] [CrossRef]
- Leroy, A.; Ribeiro, S.; Grossiord, C.; Alves, A.; Vestberg, R.H.; Salles, V.; Brunon, C.; Gritsch, K.; Grosgogeat, B.; Bayon, Y. FTIR microscopy contribution for comprehension of degradation mechanisms in PLA-based implantable medical devices. J. Mater. Sci. Mater. Med. 2017, 28, 87. [Google Scholar] [CrossRef] [PubMed]
- Langueh, C.; Changotade, S.; Ramtani, S.; Lutomski, D.; Rohman, G. Combination of in Vitro Thermally-Accelerated Ageing and Fourier-Transform Infrared Spectroscopy to Predict Scaffold Lifetime. Polym. Degrad. Stab. 2021, 183, 109454. [Google Scholar] [CrossRef]




















| Samples | Length (mm) | Width (mm) | Height (mm) | Strand (mm) | Mass (gr) | Printability |
|---|---|---|---|---|---|---|
| Sample 1 | 18.121 ± 0.037 | 18.332± | 4.061 ± 0.020 | 0.493 ± 0.003 | 0.919 ± 0.023 | 1.202 ± 0.035 |
| Sample 2 | 17.793 ± 0.023 | 18.015± | 3.465 ± 0.022 | 0.484 ± 0.002 | 0.860 ± 0.012 | 1.180 ± 0.019 |
| Sample 3 | 17.924 ± 0.031 | 17.777± | 3.791 ± 0.019 | 0.489 ± 0.002 | 0.854 ± 0.007 | 1.192 ± 0.017 |
| Sample 4 | 16.767 ± 0.015 | 16.597± | 3.374 ± 0.011 | 0.471 ± 0.001 | 0.693 ± 0.009 | 1.148 ± 0.011 |
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Response 1 | Response 2 | |
|---|---|---|---|---|---|---|---|
| Run | A: Alginate | B: Time (CrossLinking) | C: Concetration (Crosslinker) | D: Media 1 | E: UV | Degradation | Swelling |
| % | min | M | - | - | Days | % | |
| 1 | 8 | 10 | 0.5 | PBS | No | 16.5 | 16.749 |
| 2 | 6 | 10 | 0.5 | DMEM | No | 16 | 9.4675 |
| 3 | 8 | 15 | 0.05 | PBS | No | 7 | 112.544 |
| 4 | 4 | 15 | 0.5 | DMEM | Yes | 13 | 0.000 |
| 5 | 8 | 5 | 0.05 | DMEM | No | 7 | 97.262 |
| 6 | 6 | 10 | 0.1 | DMEM | No | 11 | 80.254 |
| 7 | 6 | 10 | 0.1 | DMEM | No | 10.5 | 85.993 |
| 8 | 6 | 15 | 0.05 | DMEM | Yes | 10 | 100.873 |
| 9 | 4 | 5 | 0.5 | PBS | Yes | 10 | 52.548 |
| 10 | 6 | 15 | 0.1 | PBS | No | 3.5 | 110.401 |
| 11 | 4 | 10 | 0.1 | DMEM | Yes | 7 | 39.036 |
| 12 | 4 | 10 | 0.5 | DMEM | Yes | 12 | 13.559 |
| 13 | 4 | 15 | 0.5 | PBS | No | 13 | 3.934 |
| 14 | 8 | 5 | 0.5 | DMEM | Yes | 16 | 23.195 |
| 15 | 4 | 5 | 0.05 | DMEM | Yes | 6 | 89.373 |
| 16 | 6 | 10 | 0.05 | PBS | Yes | 4.5 | 95.019 |
| 17 | 8 | 15 | 0.1 | DMEM | Yes | 15 | 82.043 |
| 18 | 4 | 10 | 0.05 | PBS | No | 2 | 88.401 |
| 19 | 6 | 10 | 0.05 | PBS | Yes | 4 | 120.275 |
| 20 | 4 | 5 | 0.5 | DMEM | No | 11 | 39.992 |
| 21 | 6 | 10 | 0.5 | PBS | Yes | 14.5 | 2.579 |
| 22 | 6 | 10 | 0.1 | DMEM | No | 11 | 91.872 |
| 23 | 6 | 5 | 0.5 | PBS | No | 13 | 25.956 |
| 24 | 8 | 15 | 0.5 | DMEM | No | 23 | 3.008 |
| 25 | 6 | 5 | 0.1 | PBS | No | 5 | 67.782 |
| 26 | 6 | 15 | 0.1 | PBS | No | 5.5 | 95.145 |
| 27 | 4 | 15 | 0.1 | PBS | Yes | 4 | 49.895 |
| 28 | 4 | 15 | 0.05 | DMEM | No | 9.5 | 71.652 |
| 29 | 8 | 15 | 0.5 | PBS | Yes | 23 | 12.945 |
| 30 | 8 | 10 | 0.1 | PBS | No | 6.5 | 105.106 |
| 31 | 6 | 15 | 0.05 | DMEM | Yes | 10.5 | 95.814 |
| 32 | 6 | 10 | 0.1 | DMEM | Yes | 10.5 | 92.279 |
| 33 | 4 | 10 | 0.1 | DMEM | Yes | 8.5 | 43.810 |
| 34 | 8 | 5 | 0.1 | PBS | Yes | 8 | 111.501 |
| Term | Standard Error * | VIF | Rᵢ2 | Power |
|---|---|---|---|---|
| A | 0.2374 | 1.12095 | 0.1079 | 98.0% |
| B | 0.2344 | 1.07312 | 0.0681 | 98.1% |
| C | 0.2290 | 1.43552 | 0.3034 | 99.6% |
| D | 0.1831 | 1.136 | 0.1197 | 99.9% |
| E | 0.1844 | 1.15616 | 0.1351 | 99.9% |
| AB | 0.2836 | 1.12591 | 0.1118 | 90.9% |
| AC | 0.2546 | 1.14246 | 0.1247 | 95.6% |
| AD | 0.2421 | 1.14503 | 0.1267 | 97.1% |
| AE | 0.2446 | 1.16822 | 0.1440 | 96.8% |
| BC | 0.2623 | 1.18698 | 0.1575 | 94.5% |
| BD | 0.2380 | 1.13322 | 0.1176 | 97.5% |
| BE | 0.2398 | 1.14976 | 0.1303 | 97.3% |
| CD | 0.1977 | 1.12462 | 0.1108 | 99.7% |
| CE | 0.1990 | 1.14333 | 0.1254 | 99.7% |
| DE | 0.1835 | 1.12884 | 0.1141 | 99.9% |
| A2 | 0.4137 | 1.40955 | 0.2906 | 99.5% |
| B2 | 0.4057 | 1.35545 | 0.2622 | 99.6% |
| C2 | 1.07 | 1.42195 | 0.2967 | 42.0% |
| Source | Alginate Ratio × Time | Alginate Ratio × Concentration | Alginate Ratio × Media | Alginate Ratio × UV Exposure | Time Alginate Ratio × UV Exposure Concentration | Time Alginate Ratio × UV Exposure Media | Time Alginate Ratio × UV Exposure UV Exposure | Concentration Alginate Ratio × UV Exposure Media | Concentration Alginate Ratio × UV Exposure UV Exposure | Media Alginate Ratio × UV Exposure UV Exposure | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terms | AB | AC | AD | AE | BC | BD | BE | CD | CE | DE | |
| p-Value | Degradation | 0.003 | 0.001 | 0.397 | 0.003 | 0.003 | 0.009 | 0.795 | 0.000 | 0.783 | 0.023 |
| Swelling | 0.143 | 0.003 | 0.879 | 0.276 | 0.050 | 0.902 | 0.126 | 0.702 | 0.999 | 0.624 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 825.460 | 11 | 75.040 | 80.010 | 0.000 |
| A-Alginate | 124.100 | 1 | 124.100 | 132.300 | 0.000 |
| B-Time (crosslinking) | 49.550 | 1 | 49.550 | 52.830 | 0.000 |
| C-Concentration (CaCl2) | 474.300 | 1 | 474.300 | 505.670 | 0.000 |
| D-Media | 74.980 | 1 | 74.980 | 79.940 | 0.000 |
| E-UV | 0.659 | 1 | 0.659 | 0.703 | 0.410 |
| AB | 11.640 | 1 | 11.640 | 12.410 | 0.001 |
| AC | 14.170 | 1 | 14.170 | 15.110 | 0.000 |
| AE | 11.680 | 1 | 11.680 | 12.450 | 0.001 |
| BC | 12.140 | 1 | 12.140 | 12.950 | 0.001 |
| BD | 7.330 | 1 | 7.330 | 7.820 | 0.010 |
| CD | 27.220 | 1 | 27.220 | 29.020 | 0.000 |
| Residual | 20.640 | 22 | 0.938 | ||
| Lack of Fit | 17.090 | 16 | 1.070 | 1.810 | 0.238 |
| Pure Error | 3.540 | 6 | 0.590 | ||
| Cor Total | 846.100 | 33 | |||
| Std. Dev. | 0.968 | R2 | 0.975 | ||
| Mean | 10.220 | Adjusted R2 | 0.963 | ||
| C.V.% | 9.480 | Predicted R2 | 0.933 | ||
| Adeq. Precision | 35.370 | ||||
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 46,294.00 | 7 | 6613.530 | 32.800 | 0.000 |
| A-Alginate | 1384.170 | 1 | 1384.170 | 6.870 | 0.014 |
| B-Time (crosslinking) | 806.930 | 1 | 806.930 | 4.000 | 0.056 |
| C-Concentration (CaCl2) | 39,930.220 | 1 | 39,930.220 | 198.050 | 0.000 |
| D-Media | 528.260 | 1 | 528.260 | 2.620 | 0.117 |
| E-UV | 13.720 | 1 | 13.720 | 0.068 | 0.796 |
| AC | 2068.080 | 1 | 2068.080 | 10.260 | 0.003 |
| BC | 1150.540 | 1 | 1150.540 | 5.710 | 0.024 |
| Residual | 5242.070 | 26 | 201.620 | ||
| Lack of Fit | 4715.090 | 20 | 235.750 | 2.680 | 0.112 |
| Pure Error | 526.980 | 6 | 87.830 | ||
| Cor Total | 51,536.770 | 33 | |||
| Std. Dev. | 14.200 | R2 | 0.898 | ||
| Mean | 62.650 | Adjusted R2 | 0.870 | ||
| C.V.% | 22.660 | Predicted R2 | 0.829 | ||
| Adeq. Precision | 18.048 | ||||
| Degradation | Swelling | ||
|---|---|---|---|
| Media | DMEM | Media | DMEM |
| UV | No | UV | No |
| +9.856 | +26.131 | ||
| −0.608 | Alginate | +10.985 | Alginate |
| −0.334 | Time (crosslinking) | +0.724 | Time (crosslinking) |
| −5.645 | Concentration (CaCl2) | +47.471 | Concentration (CaCl2) |
| +0.095 | Alginic × Time (crosslinking) | −24.374 | Alginic × Concentration (CaCl2) |
| +2.050 | Alginic × Concentration (CaCl2) | −7.390 | Time (crosslinking) × Concentration (CaCl2) |
| +0.781 | Time (crosslinking) × Concentration (CaCl2) | ||
| Media | DMEM | Media | DMEM |
| UV | Yes | UV | Yes |
| +5.331 | +24.838 | ||
| +0.194 | Alginate | +10.985 | Alginate |
| −0.334 | Time (crosslinking) | +0.724 | Time (crosslinking) |
| −5.645 | Concentration (CaCl2) | +47.471 | Concentration (CaCl2) |
| +0.095 | Alginic × Time (crosslinking) | −24.374 | Alginic × Concentration (CaCl2) |
| +2.050 | Alginic × Concentration (CaCl2) | −7.390 | Time (crosslinking) × Concentration (CaCl2) |
| +0.781 | Time (crosslinking) × Concentration (CaCl2) | ||
| Media | PBS | Media | PBS |
| UV | No | UV | No |
| +6.744 | +34.178 | ||
| −0.608 | Alginate | +10.985 | Alginate |
| −0.585 | Time (crosslinking) | +0.724 | Time (crosslinking) |
| +3.440 | Concentration (CaCl2) | +47.471 | Concentration (CaCl2) |
| +0.095 | Alginic × Time (crosslinking) | −24.374 | Alginic × Concentration (CaCl2) |
| +2.050 | Alginic × Concentration (CaCl2) | −7.390 | Time (crosslinking) × Concentration (CaCl2) |
| +0.781 | Time (crosslinking) × Concentration (CaCl2) | ||
| Media | PBS | Media | PBS |
| UV | Yes | UV | Yes |
| +2.219 | +32.885 | ||
| +0.194 | Alginate | +10.985 | Alginate |
| −0.585 | Time (crosslinking) | +0.724 | Time (crosslinking) |
| +3.440 | Concentration (CaCl2) | +47.471 | Concentration (CaCl2) |
| +0.095 | Alginic × Time (crosslinking) | −24.374 | Alginic × Concentration (CaCl2) |
| +2.050 | Alginic × Concentration (CaCl2) | −7.390 | Time (crosslinking) × Concentration (CaCl2) |
| +0.781 | Time (crosslinking) × Concentration (CaCl2) | ||
| Response | Predicted Mean | Predicted Median | Observed | Std. Dev | SE Mean | 95% CI Low for Mean | 95% CI High for Mean | 95% TI Low for 99% Pop | 95% TI High for 99% Pop |
|---|---|---|---|---|---|---|---|---|---|
| Degradation | 12.990 | 12.990 | 14.000 | 0.968 | 0.543 | 11.866 | 14.115 | 8.725 | 17.252 |
| Swelling | 97.813 | 97.813 | 93.872 | 14.199 | 6.587 | 84.272 | 111.355 | 39.055 | 156.52 |
| Factors | A: Alginate Ratio (%) | B: Time (Crosslinking)(min) | C: Concentration (CaCl2) (M) | D: Media (-) | E: UV Exposure (-) |
|---|---|---|---|---|---|
| Optimal Levels | 8.000 | 15.000 | 0.284 | DMEM | Yes |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | ||
|---|---|---|---|---|---|---|---|
| Degradation Analysis | Design Expert | Model | 825.460 | 11 | 75.040 | 80.010 | 0.000 |
| Minitab | 825.460 | 11 | 75.040 | 80.010 | 0.000 | ||
| Swelling ratio Analysis | Design Expert | Model | 46,294.700 | 7 | 6613.530 | 32.800 | 0.000 |
| Minitab | 46,294.700 | 7 | 6613.530 | 32.800 | 0.000 | ||
| Degradation Analysis | Design Expert | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | ||
| Minitab | 0.968 | 0.975 | 0.963 | 0.933 | |||
| Swelling ratio Analysis | Design Expert | 0.968 | 0.975 | 0.963 | 0.933 | ||
| Minitab | 14.200 | 0.898 | 0.870 | 0.829 | |||
| Optimal Response | Predicted Mean | Predicted Median | Observed | Std Dev | SE Mean | 95% CI Low for Mean | 95% CI High for Mean | 95% TI Low for 99% Pop | 95% TI High for 99% Pop |
|---|---|---|---|---|---|---|---|---|---|
| Degradation | 19.654 | 19.654 | 18.500 | 0.968 | 0.607 | 18.376 | 20.897 | 15.262 | 24.011 |
| Swelling | 50.00 | 50.000 | 54.120 | 14.189 | 6.502 | 36.840 | 63.571 | −8.406 | 108.818 |
| Post-Printing Treatment Factor | A: Alginate Ratio (%) | B: Time (CrossLinking) (min) | C: Concentration (CrossLinker) (M) |
|---|---|---|---|
| Low Level | 4 | 5 | 0.05 |
| Medium Level | 6 | 10 | 0.1 |
| High Level | 8 | 15 | 0.5 |
| Post-Printing Treatment Factors | D: Culture Media | E: UV Exposure |
|---|---|---|
| Level 1 | DMEM | NO |
| Level 2 | PBS | YES |
| Post-Printing Treatment Factors | A: Alginate Ratio (%) | B: Time (Crosslinking) (min) | C: Concentration (CaCl2) (M) | D: Media (-) | E: UV Exposure (-) |
|---|---|---|---|---|---|
| Sample 1 | 4 | 5 | 0.05 | PBS | No |
| Sample 2 | 6 | 10 | 0.1 | PBS | Yes |
| Sample 3 | 6 | 10 | 0.1 | PBS | No |
| Sample 4 | 8 | 15 | 0.5 | DMEM | Yes |
| Hydrogel | Alginate (%) | Gelatin (%) |
|---|---|---|
| 1 | 4 | 4 |
| 2 | 6 | 4 |
| 3 | 8 | 4 |
| Printing Parameters | Temperature (˚C) | Extrusion Speed (mm/s) | Nozzle Diameter (mm) | Layer Height (mm) | Perimeter Speed (mm/s) | Infill Speed (mm/s) | Retract Speed (mm/s) | Travel Speed (mm/s) |
|---|---|---|---|---|---|---|---|---|
| Printability Window | 24 | 2 | 0.41 | 0.25 | 3 | 2 | 30 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. https://doi.org/10.3390/gels9110857
Kaliampakou C, Lagopati N, Pavlatou EA, Charitidis CA. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels. 2023; 9(11):857. https://doi.org/10.3390/gels9110857
Chicago/Turabian StyleKaliampakou, Christina, Nefeli Lagopati, Evangelia A. Pavlatou, and Costas A. Charitidis. 2023. "Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior" Gels 9, no. 11: 857. https://doi.org/10.3390/gels9110857
APA StyleKaliampakou, C., Lagopati, N., Pavlatou, E. A., & Charitidis, C. A. (2023). Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels, 9(11), 857. https://doi.org/10.3390/gels9110857