Akkermansia muciniphila Encapsulated in Calcium-Alginate Hydrogelated Matrix: Viability and Stability over Aerobic Storage and Simulated Gastrointestinal Conditions
Abstract
:1. Introduction
2. Results and Discussion
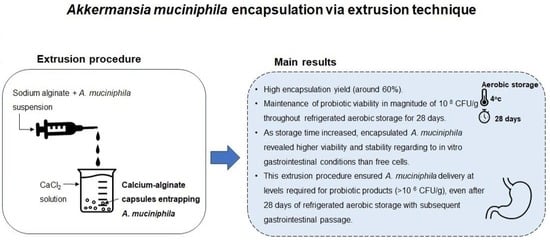
2.1. Capsules Morphology and Encapsulation Yield of Calcium-Alginate Capsules Entrapping Akkermansia muciniphila
2.2. Viability of Akkermansia muciniphila in Encapsulated and Free Forms during Refrigerated Aerobic Storage
2.3. Survival of Akkermansia muciniphila in Encapsulated and Free Forms When Exposed to In Vitro Simulated Gastrointestinal Conditions
3. Conclusions
4. Materials and Methods
4.1. Bacterial Strain and Culture Conditions
4.2. Extrusion Procedure
4.3. Enumeration of Free and Encapsulated Akkermansia muciniphila Cells
4.4. Encapsulation Yield Calculation
4.5. Capsules Morphology
4.6. Viability of Free and Encapsulated Akkermansia muciniphila Cells during Refrigerated Aerobic Storage
4.7. Survival of Free and Encapsulated Akkermansia muciniphila Cells in In Vitro Simulated Gastrointestinal Passage
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A Taxonomic Note on the Genus Lactobacillus: Description of 23 Novel Genera, Emended Description of the Genus Lactobacillus beijerinck 1901, and Union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- El Hage, R.; Hernandez-Sanabria, E.; Van de Wiele, T. Emerging Trends in “Smart Probiotics”: Functional Consideration for the Development of Novel Health and Industrial Applications. Front. Microbiol. 2017, 8, 1889. [Google Scholar] [CrossRef] [PubMed]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to Select a Probiotic? A Review and Update of Methods and Criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Municiphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; De Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Pellegrino, A.; Coppola, G.; Santopaolo, F.; Gasbarrini, A.; Ponziani, F.R. Role of Akkermansia in Human Diseases: From Causation to Therapeutic Properties. Nutrients 2023, 15, 1815. [Google Scholar] [CrossRef]
- Koh, W.Y.; Lim, X.X.; Tan, T.-C.; Kobun, R.; Rasti, B. Encapsulated Probiotics: Potential Techniques and Coating Materials for Non-Dairy Food Applications. Appl. Sci. 2022, 12, 10005. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Chandramouli, V.; Kailasapathy, K.; Peiris, P.; Jones, M. An Improved Method of Microencapsulation and Its Evaluation to Protect Lactobacillus Spp. in Simulated Gastric Conditions. J. Microbiol. Methods 2004, 56, 27–35. [Google Scholar] [CrossRef]
- Sousa, S.; Gomes, A.M.; Pintado, M.M.; Malcata, F.X.; Silva, J.P.; Sousa, J.M.; Costa, P.; Amaral, M.H.; Rodrigues, D.; Rocha-santos, T.A.P.; et al. Encapsulation of Probiotic Strains in Plain or Cysteine-Supplemented Alginate Improves Viability at Storage below Freezing Temperatures. Eng. Life Sci. 2012, 12, 457–465. [Google Scholar] [CrossRef]
- Amine, K.M.; Champagne, C.P.; Salmieri, S.; Britten, M.; St-Gelais, D.; Fustier, P.; Lacroix, M. Effect of Palmitoylated Alginate Microencapsulation on Viability of Bifidobacterium Longum during Freeze-Drying. LWT Food Sci. Technol. 2014, 56, 111–117. [Google Scholar] [CrossRef]
- Frakolaki, G.; Giannou, V.; Topakas, E.; Tzia, C. Effect of Various Encapsulating Agents on the Beads’ Morphology and the Viability of Cells during BB-12 Encapsulation through Extrusion. J. Food Eng. 2021, 294, 110423. [Google Scholar] [CrossRef]
- Van der Ark, K.C.H.; Nugroho, A.D.W.; Berton-Carabin, C.; Wang, C.; Belzer, C.; de Vos, W.M.; Schroen, K. Encapsulation of the Therapeutic Microbe Akkermansia muciniphila in a Double Emulsion Enhances Survival in Simulated Gastric Conditions. Food Res. Int. 2017, 102, 372–379. [Google Scholar] [CrossRef]
- Marcial-Coba, M.S.; Cieplak, T.; Cahú, T.B.; Blennow, A.; Knøchel, S.; Nielsen, D.S. Viability of Microencapsulated Akkermansia muciniphila and Lactobacillus plantarum during Freeze-Drying, Storage and in Vitro Simulated Upper Gastrointestinal Tract Passage. Food Funct. 2018, 9, 5868–5879. [Google Scholar] [CrossRef]
- Almeida, D.; Machado, D.; Sousa, S.; Seabra, C.L.; Barbosa, J.C.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Effect of Emulsification/Internal Gelation-Based Microencapsulation on the Viability of Akkermansia muciniphila upon Prolonged Storage and Simulated Gastrointestinal Passage. Food Hydrocoll. Health 2022, 2, 100084. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Barbosa, J.; Almeida, D.; Machado, D.; Sousa, S.; Freitas, A.; Andrade, J.; Gomes, A. Spray-Drying Encapsulation of the Live Biotherapeutic Candidate Akkermansia muciniphila DSM 22959 to Survive Aerobic Storage. Pharmaceuticals 2022, 15, 628. [Google Scholar] [CrossRef]
- Chang, Y.; Yang, Y.; Xu, N.; Mu, H.; Zhang, H.; Duan, J. Improved Viability of Akkermansia muciniphila by Encapsulation in Spray Dried Succinate-Grafted Alginate Doped with Epigallocatechin-3-Gallate. Int. J. Biol. Macromol. 2020, 159, 373–382. [Google Scholar] [CrossRef]
- Machado, D.; Almeida, D.; Seabra, C.L.; Andrade, J.C.; Gomes, A.M.; Freitas, A.C. Uncovering Akkermansia muciniphila Resilience or Susceptibility to Different Temperatures, Atmospheres and Gastrointestinal Conditions. Anaerobe 2020, 61, 102135. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.M.; Hill, C. The Interaction between Bacteria and Bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.A. Host Immunostimulation and Substrate Utilization of the Gut Symbiont Akkermansia muciniphila; Wageningen University: Wageningen, The Netherlands, 2015. [Google Scholar]
- Tan, L.L.; Ang, K.L.; Loo, S.C.J. Alginate Encapsulation Improves Probiotics Survival in Carbonated Sodas and Beers. PLoS ONE 2023, 18, e0283745. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, M.; You, X.; Sela, D.A.; Xiao, H.; McClements, D.J. Encapsulation of Bifidobacterium in Alginate Microgels Improves Viability and Targeted Gut Release. Food Hydrocoll. 2021, 116, 106634. [Google Scholar] [CrossRef]
- Chen, S.-C.; Wu, Y.-C.; Mi, F.-L.; Lin, Y.-H.; Yu, L.-C.; Sung, H.-W. A Novel PH-Sensitive Hydrogel Composed of N,O-Carboxymethyl Chitosan and Alginate Cross-Linked by Genipin for Protein Drug Delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar] [CrossRef] [PubMed]
- DSMZ Akkermansia muciniphila DSM 22959. Available online: https://www.dsmz.de/collection/catalogue/details/culture/DSM-22959 (accessed on 22 May 2023).
- Martin, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Effect of Unmodified Starch on Viability of Alginate-Encapsulated Lactobacillus fermentum CECT5716. LWT Food Sci. Technol. 2013, 53, 480–486. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]


| Condition | Day 1 | Day 28 | ||
|---|---|---|---|---|
| Encapsulated | Free | Encapsulated | Free | |
| Initial Concentration | (3.82 ± 1.26) × 108 | (3.40 ± 0.39) × 109 | (2.32 ± 0.55) × 108 | (6.12 ± 1.11) × 108 |
| After gastric phase | (2.74 ± 1.17) × 108 | (2.57 ± 0.31) × 1010 | (1.15 ± 0.12) × 108 | (7.46 ± 6.91) × 106 |
| After intestinal phase | (1.57 ± 0.33) × 108 | (4.83 ± 0.36) × 109 | (2.93 ± 1.09) × 107 | <LOD 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, D.; Fonseca, M.; Vedor, R.; Sousa, S.; Barbosa, J.C.; Gomes, A.M. Akkermansia muciniphila Encapsulated in Calcium-Alginate Hydrogelated Matrix: Viability and Stability over Aerobic Storage and Simulated Gastrointestinal Conditions. Gels 2023, 9, 869. https://doi.org/10.3390/gels9110869
Machado D, Fonseca M, Vedor R, Sousa S, Barbosa JC, Gomes AM. Akkermansia muciniphila Encapsulated in Calcium-Alginate Hydrogelated Matrix: Viability and Stability over Aerobic Storage and Simulated Gastrointestinal Conditions. Gels. 2023; 9(11):869. https://doi.org/10.3390/gels9110869
Chicago/Turabian StyleMachado, Daniela, Mariana Fonseca, Rita Vedor, Sérgio Sousa, Joana Cristina Barbosa, and Ana Maria Gomes. 2023. "Akkermansia muciniphila Encapsulated in Calcium-Alginate Hydrogelated Matrix: Viability and Stability over Aerobic Storage and Simulated Gastrointestinal Conditions" Gels 9, no. 11: 869. https://doi.org/10.3390/gels9110869
APA StyleMachado, D., Fonseca, M., Vedor, R., Sousa, S., Barbosa, J. C., & Gomes, A. M. (2023). Akkermansia muciniphila Encapsulated in Calcium-Alginate Hydrogelated Matrix: Viability and Stability over Aerobic Storage and Simulated Gastrointestinal Conditions. Gels, 9(11), 869. https://doi.org/10.3390/gels9110869