A Study on Enhanced Electrorheological Performance of Plate-like Materials via Percolation Gel-like Effect
Abstract
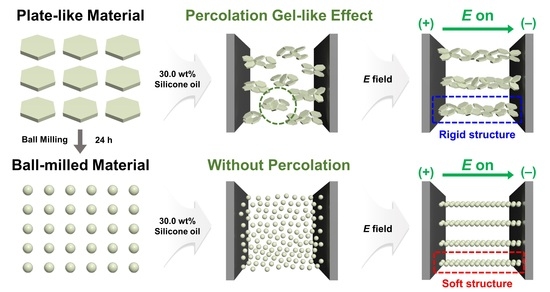
:1. Introduction
2. Results and Discussion
2.1. Characterization of NM/BM24h, SM/BM24h, and GL/BM24h Materials
2.2. Concentration Dependence of the ER Fluids at Zero-Field Condition
2.3. ER Activities of NM-, SM-, and GL-Based ER Fluids
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of NM/BM24h, SM/BM24h, and GL/BM24h
4.3. Characterization
4.4. Investigation of ER Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, C.-M.; Jang, Y.; Lee, S.; Jang, J. Dual electric and magnetic responsivity of multilayered magnetite-embedded core/shell silica/titania nanoparticles with outermost silica shell. J. Mater. Chem. C 2018, 6, 10241. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Hui, X.; Huang, Y.; Chen, J.; Hu, C.; Guo, H.; Qi, S.; Wang, Z.L. Self-powered In-Phase Sensing and Regulating Mechanical System Enabled by Nanogenerator and Electrorheological Fluid. Adv. Funct. Mater. 2022, 33, 2212248. [Google Scholar] [CrossRef]
- Linzhi, L.I.; Shujuan, G.A.O. Polyaniline (PANI) and BaTiO3 composite nanotube with high suspension performance in electrorheological fluid. Mater. Today Commun. 2020, 24, 100993. [Google Scholar] [CrossRef]
- Vu, C.M.; Nguyen, V.-H.; Bach, Q.-V. Influences of electric field strength on rheological properties of electrorheological fluid based on hollow poly (O-phenylenediamine co O-toluidine) dispersed on silicone oil. J. Mol. Liq. 2020, 314, 113762. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Choi, K.; Choi, H.J. Novel post-treatment of removed fine dust particle: Electrorheological application. J. Clean. Prod. 2022, 368, 133128. [Google Scholar] [CrossRef]
- Huo, X.; Yossifon, G. Tunable Electrorheological Fluid Microfluidic Rectifier: Irreversibility of Viscous Flow Due to Spatial Asymmetry Induced Memory Effects. Phys. Rev. Lett. 2019, 123, 194502. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Cho, K.H.; Jang, Y.; Kim, J.; Lee, K.; Yu, H.; Lee, S.; Jang, J. Synthesis and Electroresponse Activity of Porous Polypyrrole/Silica−Titania Core/Shell Nanoparticles. Langmuir 2018, 34, 15773–15782. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Seo, Y.; Choi, H.J. Recent development of electro-responsive smart electrorheological fluids. Soft Matter 2019, 15, 3473–3486. [Google Scholar] [CrossRef]
- Choi, K.; Gao, C.Y.; Nam, J.D.; Choi, H.J. Cellulose-Based Smart Fluids under Applied Electric Fields. Materials 2017, 10, 1060. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, Y.; Lei, Q.; Zhao, X.; Yin, J. Influence of particle size on electrorheological effect of poly(ionic liquid) microsphere suspensions. Colloids Surf. A Physicochem. Eng. 2023, 672, 131745. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Jang, Y.; Noh, J.; Kim, J.; Lee, K.; Jang, J. Enhanced Electrorheological Performance of Mixed Silica Nanomaterial Geometry. ACS Appl. Mater. 2017, 9, 36358–36367. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wen, X.; Liu, Z.; Wang, J.; Zhang, P. Synthesis and electrorheological performances of 2D PANI/TiO2 nanosheets. Colloids Surf. A Physicochem. 2018, 552, 24–31. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Lee, K.; Noh, J.; Lee, S.; Jang, J. Electrorheological performance of multigram-scale mesoporous silica particles with different aspect ratios. J. Mater. Chem. C 2016, 4, 1713–1719. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Choi, M.; Kim, C.; Jang, J. Geometrical study of electrorheological activity with shape-controlled titania-coated silica nanomaterials. J. Colloid Interface Sci. 2010, 347, 177–182. [Google Scholar] [CrossRef]
- Wu, J.; Xu, G.; Cheng, Y.; Liu, F.; Guo, J.; Cui, P. The influence of high dielectric constant core on the activity of core–shell structure electrorheological fluid. J. Colloid Interface Sci. 2012, 378, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Wang, L.; Bai, Q.; Wang, B.; Hao, C. Synthesis and electrorheological properties of TiOF2 @SiO2 cubic-like core/shell nanocomposite. J. Mol. Liq. 2022, 366, 120335. [Google Scholar] [CrossRef]
- Jing, H.; Hua, L.; Long, F.; Lv, B.; Wang, B.; Zhang, H.; Fan, X.; Zheng, H.; Chu, C.; Xu, G.; et al. Variable stiffness and fast-response soft structures based on electrorheological fluids. J. Mater. Chem. C 2023, 11, 11842–11850. [Google Scholar] [CrossRef]
- Sun, G.; Lu, K.; Kun, F. Percolation-induced conductor-insulator transition in a system of metal spheres in a dielectric fluid. Phys. Rev. E 2011, 83, 041405. [Google Scholar] [CrossRef]
- Tsuda, K.; Hirose, Y.; Ogura, H.; Otsubo, Y. Motion control of pattern electrode by electrorheological fluids. Colloids Surf. A Physicochem. 2010, 360, 57–62. [Google Scholar] [CrossRef]
- Peddireddy, K.R.; Capron, I.; Nicolai, T.; Benyahia, L. Gelation Kinetics and Network Structure of Cellulose Nanocrystals in Aqueous Solution. Biomacromolecules 2016, 17, 3298–3304. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Derkach, S.R.; Kulichikhin, V.G. Rheology of Gels and Yielding Liquids. Gels 2023, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Choi, H.J.; Seo, Y. Analysis of the flow behavior of electrorheological fluids with the aligned structure reformation. Polymer 2011, 52, 5695–5698. [Google Scholar] [CrossRef]
- Yoon, C.-M.; Noh, J.; Jang, Y.; Jang, J. Fabrication of a silica/titania hollow nanorod and its electroresponsive activity. RSC Adv. 2017, 7, 19754–19763. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.-M.; Jang, J. Enhanced electrorheological activity of polyaniline coated mesoporous silica with high aspect ratio. J. Colloid Interface Sci. 2016, 470, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gadige, P.; Bandyopadhyay, R. Electric field induced gelation in aqueous nanoclay suspensions. Soft Matter 2018, 14, 6974–6982. [Google Scholar] [CrossRef] [PubMed]
- Kutalkova, E.; Plachy, T.; Sedlacik, M. On the enhanced sedimentation stability and electrorheological performance of intelligent fluids based on sepiolite particles. J. Mol. Liq. 2020, 309, 113120. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Saleh, S.A.A.; Abdel-Hameed, S.A.M.; Fayad, A.M. Catalytic, kinetic and thermodynamic properties of free and immobilized caseinase on mica glass-ceramics. Heliyon 2019, 5, e01674. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, S.; Singh, L.; Lochab, S.P. Oxygen ion-induced modifications of optical properties of natural muscovite mica. Radiat. Eff. Defects Solids 2013, 168, 587–593. [Google Scholar] [CrossRef]
- Ohta, S.-I. Synthetic Mica and Its Application. Clay Sci. 2006, 12, 119–124. [Google Scholar]
- He, Y.; Zhou, B. The Influence of Mica Iron Oxide Pigments on Epoxy Coating Properties Prepared on AISI 1045 Steel Rebar Immersed in 3.5 wt% NaCl Solution. Int. J. Electrochem. Sci. 2021, 16, 210726. [Google Scholar] [CrossRef]
- Jekal, S.; Kim, J.; Lu, Q.; Kim, D.-H.; Noh, J.; Kim, H.-Y.; Kim, M.-J.; Kim, M.-S.; Oh, W.-C.; Choi, H.-J.; et al. Development of Novel Colorful Electrorheological Fluids. Nanomaterials 2022, 12, 3113. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, S.V. Studies on contact interaction of mica and glass. IOP Conf. Ser. Mater. Sci. Eng. 2019, 687, 066055. [Google Scholar] [CrossRef]
- Singh, A.K.; Rajak, P.K.; Pati, J.K.; Dwivedi, M.M.; Pandey, K.; Prakash, K.; Rai, A.K.; Dwivedi, S. Experimental Synthesis of Coloured Soda-lime-silica (SLS) Glasses using Untreated Silica Sand of Shankargarh Area (Prayagraj District, Uttar Pradesh, India) and its Ramifications. J. Geol. Soc. India 2022, 98, 740–748. [Google Scholar] [CrossRef]
- Vancea, C.; Lazău, I. Glass foam from window panes and bottle glass wastes. Cent. Eur. J. Chem. 2014, 12, 804–811. [Google Scholar] [CrossRef]
- Guan, W.; Ji, F.; Chen, Q.; Yan, P.; Pei, L. Synthesis and Enhanced Phosphate Recovery Property of Porous Calcium Silicate Hydrate Using Polyethyleneglycol as Pore-Generation Agent. Materials 2013, 6, 2846–2861. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, G.; Prabakaran, A.R.; Gunasekaran, S. Spectroscopic Characterization of Indian Standard Sand. J. Appl. Spectrosc. 2010, 77, 86–94. [Google Scholar] [CrossRef]
- Barakat, M.A.; Selim, A.Q.; Mobarak, M.; Kumar, R.; Anastopoulos, I.; Giannakoudakis, D.; Bonilla-Petriciolet, A.; Mohamed, E.A.; Seliem, M.K.; Komarneni, S. Experimental and Theoretical Studies of Methyl Orange Uptake by Mn–Rich Synthetic Mica: Insights into Manganese Role in Adsorption and Selectivity. Nanomaterials 2020, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhang, X.; Zhu, L.; Li, X.; Meng, L. Synthesis and characterization of mesoporous alumina, and adsorption performance for n-butane. Res. Chem. Intermed. 2015, 41, 3637–3648. [Google Scholar] [CrossRef]
- Fali, A.; Gamage, S.; Howard, M.; Folland, T.G.; Mahadik, N.A.; Tiwald, T.; Bolotin, K.; Caldwell, J.D.; Abate, Y. Nanoscale Spectroscopy of Dielectric Properties of Mica. ACS Photonics 2021, 8, 175–181. [Google Scholar] [CrossRef]
- Nakanishi, H.; Nagahiro, S.-I.; Mitarai, N. Fluid dynamics of dilatant fluids. Phys. Rev. E 2012, 85, 011401. [Google Scholar] [CrossRef]
- Piednoir, A.; Steinberger, A.; Cottin-Bizonne, C.; Barentin, C. Apparent Non-Newtonian Behavior of Ionic Liquids. J. Phys. Chem. B 2020, 124, 2685–2690. [Google Scholar] [CrossRef]
- Jyoti, B.V.S.; Baek, S.W. Rheological Characterization of Hydrogen Peroxide Gel Propellant. Int. J. Aeronaut. Space 2014, 15, 199–204. [Google Scholar] [CrossRef]
- Mirigliano, M.; Borghi, F.; Podestà, A.; Antidormi, A.; Colombo, L.; Milani, P. Non-ohmic behavior and resistive switching of Au cluster-assembled films beyond the percolation threshold. Nanoscale Adv. 2019, 1, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Nair, G.G.; Prasad, S.K.; Bhargavi, R.; Jayalakshmi, V.; Shanker, G.; Yelamaggad, C.V. Soft glass rheology in liquid crystalline gels formed by a monodisperse dipeptide. J. Phys. Chem. B 2010, 114, 697–704. [Google Scholar] [CrossRef]
- Horváth, B.; Guba, S.; Balogh, D.; Jakab, M.; Szalai, I. Effect of percolation on the deformation of isotropic electrorheological elastomers under external electric fields. J. Mol. Liq. 2023, 390, 123046. [Google Scholar] [CrossRef]
- Kaneko, K.; Ujihara, Y.; Oto, K.; Hashishin, T.; Hanasaki, T. Electric-Field-Induced Viscosity Change of a Nematic Liquid Crystal with Gold Nanoparticles. ChemPhysChem 2015, 16, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Dhar, J.; Ghosh, U.; Chakraborty, S. Electro-capillary effects in capillary filling dynamics of electrorheological fluids. Soft Matter 2015, 11, 6957–6967. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, L.; Xin, X.; Zhang, Y.; Wang, H.; Sun, A.; Cheng, Y.; Chen, X.; Xu, G. Electrorheological Fluids with High Shear Stress Based on Wrinkly Tin Titanyl Oxalate. ACS Appl. Mater. 2018, 10, 6785–6792. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, Y.; Zheng, H.; Li, C.; Ma, L.; Zhang, H.; Wang, B.; Hao, C. Composites of Co-Doped Graphitic C3N4 Nanosheets and TiO2 Nanoparticles for Electrorheological Fluid Applications. ACS Appl. Nano Mater. 2022, 5, 1003–1015. [Google Scholar] [CrossRef]
- Zhang, W.L.; Jiang, D.; Wang, X.; Hao, B.N.; Liu, Y.D.; Liu, J. Growth of Polyaniline Nanoneedles on MoS2 Nanosheets, Tunable Electroresponse, and Electromagnetic Wave Attenuation Analysis. J. Phys. Chem. C 2017, 121, 4989–4998. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.; Cho, J.-C.; Lim, H.-S.; Kim, J.W.; Suh, K.-D. Monodisperse conducting colloidal dipoles with symmetric dimer structure for enhancing electrorheology properties. J. Colloid Interface Sci. 2012, 374, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Hong, S.; Yoon, C.-M.; Lee, S.; Jang, J. Dual external field-responsive polyaniline-coated magnetite/silica nanoparticles for smart fluid applications. Chem. Commun. 2017, 53, 6645. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.S.; Kwon, S.H.; Lee, J.H.; Choi, H.J. Facile fabrication of core-shell typed silica/poly(diphenylamine) composite microparticles and their electro-response. Polymer 2019, 182, 121851. [Google Scholar] [CrossRef]
- Ghannam, M.T.; Selim, M.Y.E.; Zekri, A.Y.; Esmrail, N. Rheological Assessment of Oil-Xanthan Emulsions in Terms of Complex, Storage, and Loss Moduli. Polymers 2023, 15, 470. [Google Scholar] [CrossRef] [PubMed]
- Zara, Y.; Rhee, K.Y. Modeling of viscosity and complex modulus for poly(lactic acid)/poly(ethylene oxide)/carbon nanotubes nanocomposites assuming yield stress and network breaking time. Compos. B. Eng. 2019, 156, 100–107. [Google Scholar] [CrossRef]








| Samples | Elemental Composition (wt%) | ||
|---|---|---|---|
| Si | Al | K | |
| NM | 44.6 | 40.6 | 14.8 |
| SM | 69.5 | 18.3 | 12.2 |
| GL | 100.0 | - b | - |
| NM/BM24h | 42.4 | 41.2 | 16.4 |
| SM/BM24h | 70.5 | 18.6 | 10.9 |
| GL/BM24h | 100.0 | - | - |
| Samples | Atomic Percentage (%) | |||
|---|---|---|---|---|
| Si | Al | K | O | |
| NM | 16.7 | 13.0 | 4.2 | 66.1 |
| SM | 19.5 | 8.6 | 6.2 | 65.7 |
| GL | 34.6 | 2.6 | 1.8 | 61.0 |
| Samples | Ionic Concentrations (ppm) | ||
|---|---|---|---|
| K+ | Na+ | Ca2+ | |
| NM | 3.5 | - b | - |
| SM | 9.7 | 20.6 | 12.5 |
| GL | - | 5.4 | 2.6 |
| NM/BM24h | 3.3 | - | - |
| SM/BM24h | 9.2 | 19.5 | 13.1 |
| GL/BM24h | - | 4.8 | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jekal, S.; Sa, M.; Chu, Y.-R.; Kim, C.-G.; Noh, J.; Kim, J.; Kim, H.-Y.; Oh, W.-C.; Otgonbayar, Z.; Yoon, C.-M. A Study on Enhanced Electrorheological Performance of Plate-like Materials via Percolation Gel-like Effect. Gels 2023, 9, 891. https://doi.org/10.3390/gels9110891
Jekal S, Sa M, Chu Y-R, Kim C-G, Noh J, Kim J, Kim H-Y, Oh W-C, Otgonbayar Z, Yoon C-M. A Study on Enhanced Electrorheological Performance of Plate-like Materials via Percolation Gel-like Effect. Gels. 2023; 9(11):891. https://doi.org/10.3390/gels9110891
Chicago/Turabian StyleJekal, Suk, Minki Sa, Yeon-Ryong Chu, Chan-Gyo Kim, Jungchul Noh, Jiwon Kim, Ha-Yeong Kim, Won-Chun Oh, Zambaga Otgonbayar, and Chang-Min Yoon. 2023. "A Study on Enhanced Electrorheological Performance of Plate-like Materials via Percolation Gel-like Effect" Gels 9, no. 11: 891. https://doi.org/10.3390/gels9110891
APA StyleJekal, S., Sa, M., Chu, Y. -R., Kim, C. -G., Noh, J., Kim, J., Kim, H. -Y., Oh, W. -C., Otgonbayar, Z., & Yoon, C. -M. (2023). A Study on Enhanced Electrorheological Performance of Plate-like Materials via Percolation Gel-like Effect. Gels, 9(11), 891. https://doi.org/10.3390/gels9110891