Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel
Abstract
:1. Introduction
2. Results and Discussion
2.1. Hydrogel Morphology by SEM
2.2. Amorphous-Crystalline Nature of Gelatins via PXRD
2.3. NMR Relaxation and Self-Diffusion
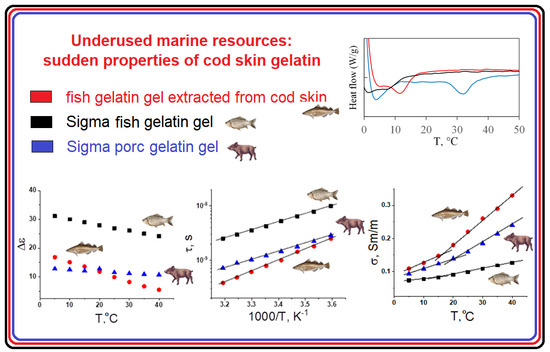
2.4. Dielectric Response of Gelatin Hydrogels
2.5. Differential Scanning Calorimetry Study of Gel Melting
2.6. Rheological Data
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Scanning Electron Microscopy
4.2.2. X-ray Powder Diffraction
4.2.3. NMR Spectroscopy
4.2.4. Dielectric Spectroscopy
4.2.5. Differential Scanning Calorimetry
4.2.6. Rheological Measurements
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haug, I.J.; Draget, K.I. Gelatin. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 142–163. [Google Scholar]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialization. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Mikhailov, O.V. Gelatin as It Is: History and Modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Sun, R.; Ding, M.; Li, L.; Tao, N.; Wang, X.; Zhong, J. Commercial cold-water fish skin gelatin and bovine bone gelatin: Structural, functional, and emulsion stability differences. LWT-Food Sci. Technol. 2020, 125, 109207. [Google Scholar] [CrossRef]
- Rather, J.A.; Akhter, N.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Khaneghah, A.M.; Dar, B.N. A comprehensive review on gelatin: Understanding impact of the sources, extraction methods, and modifications on potential packaging applications. Food Packag. Shelf Life 2022, 34, 100945. [Google Scholar] [CrossRef]
- Lee, C.H.; Chin, K.B. Effect of Pork Skin Gelatin on the Physical Properties of Pork Myofibrillar Protein Gel and Restructured Ham with Microbial Transglutaminase. Gels 2022, 8, 822. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Prokopová, A.; Gál, R.; Mokrejš, P.; Pavlačková, J. Preparation of Gelatin from Broiler Chicken Stomach Collagen. Foods 2023, 12, 127. [Google Scholar] [CrossRef]
- Lv, L.-C.; Huang, Q.-Y.; Ding, W.; Xiao, X.-H.; Zhang, H.-Y.; Xiong, L.-X. Fish gelatin: The novel potential applications. J. Funct. Foods 2019, 63, 103581. [Google Scholar] [CrossRef]
- Nitsuwat, S.; Zhang, P.; Ng, K.; Fang, Z. Fish gelatin as an alternative to mammalian gelatin for food industry: A meta-analysis. LWT-Food Sci. Technol. 2021, 141, 110899. [Google Scholar] [CrossRef]
- Athanasopoulou, E.; Michailidi, A.; Ladakis, D.; Kalliampakou, K.I.; Flemetakis, E.; Koutinas, A.; Tsironi, T. Extraction of Fish Protein Concentrates from Discards and Combined Application with Gelatin for the Development of Biodegradable Food Packaging. Sustainability 2023, 15, 12062. [Google Scholar] [CrossRef]
- Rocha-Pimienta, J.; Navajas-Preciado, B.; Barraso-Gil, C.; Martillanes, S.; Delgado-Adámez, J. Optimization of the Extraction of Chitosan and Fish Gelatin from Fishery Waste and Their Antimicrobial Potential as Active Biopolymers. Gels 2023, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Du, Z. Seafood Waste-Based Materials for Sustainable Food Packing: From Waste to Wealth. Sustainability 2022, 14, 16579. [Google Scholar] [CrossRef]
- Zarubin, N.Y.; Kharenko, E.N.; Bredikhina, O.V.; Arkhipov, L.O.; Zolotarev, K.V.; Mikhailov, A.N.; Nakhod, V.I.; Mikhailova, M.V. Application of the Gadidae Fish Processing Waste for Food Grade Gelatin Production. Mar. Drugs 2021, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Iskakov, R.; Sugirbay, A. Technologies for the Rational Use of Animal Waste: A Review. Sustainability 2023, 15, 2278. [Google Scholar] [CrossRef]
- Fratzl, P. Collagen: structure and mechanics, an introduction. In Collagen: Structure and Mechanics; Fratzl, P., Ed.; Springer US: Boston, MA, USA, 2008; pp. 1–13. [Google Scholar]
- Yang, M.; Yang, L.; Xu, J.; Nie, Y.; Wu, W.; Zhang, T.; Wang, X.; Zhong, J. Comparison of silver carp fin gelatins extracted by three types of methods: Molecular characteristics, structure, function, and pickering emulsion stabilization. Food Chem. 2022, 368, 130818. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zi, Y.; Xu, J.; Zheng, Y.; Huang, S.; Hu, Y.; Liu, B.; Wang, X.; Zhong, J. Effect of extraction methods on the properties of tilapia scale gelatins. Int. J. Biol. Macromol. 2022, 221, 1150–1160. [Google Scholar] [CrossRef]
- Said, N.S.; Sarbon, N.M. Physical and Mechanical Characteristics of Gelatin-Based Films as a Potential Food Packaging Material: A Review. Membranes 2022, 12, 442. [Google Scholar] [CrossRef]
- Schrieber, R.; Gareis, H. Gelatine Handbook; Wiley-VCH GmbH & Co: Weinhem, Germany, 2007. [Google Scholar]
- Brodsky, B.; Persikov, A.V. Molecular structure of the collagen triple helix. Adv. Protein Chem. 2005, 70, 301–339. [Google Scholar]
- Steyaert, I.; Rahier, H.; Van Vlierberghe, S.; Olijve, J.; De Clerck, K. Gelatin nanofibers: Analysis of triple helix dissociation temperature and cold-water-solubility. Food Hydrocoll. 2016, 57, 200–208. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S. Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies. Polymers 2020, 12, 3051. [Google Scholar] [CrossRef]
- Valcarcel, J.; Hermida-Merino, C.; Piñeiro, M.M.; Hermida-Merino, D.; Vázquez, J.A. Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture. Int. J. Mol. Sci. 2021, 22, 12104. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xiao, J.; Zhu, M.; Yang, H.; Liu, J.; Liu, Y. Study of Physicochemical and Gelation Properties of Fish Gelatin from Different Sources. Appl. Sci. 2023, 13, 5337. [Google Scholar] [CrossRef]
- Huang, T.; Tu, Z.; Shangguan, X.; Sha, X.; Wang, H.; Zhang, L.; Bansal, N. Fish gelatin modifications: A comprehensive review. Trends Food Sci Technol. 2019, 86, 260–269. [Google Scholar] [CrossRef]
- Lin, L.; Regenstein, J.M.; Lv, S.; Lu, J.; Jiang, S. An overview of gelatin derived from aquatic animals: Properties and modification. Trends Food Sci Technol. 2017, 68, 102–112. [Google Scholar] [CrossRef]
- Ahmad Hariza, A.M.; Mohd Yunus, M.H.; Fauzi, M.B.; Murthy, J.K.; Tabata, Y.; Hiraoka, Y. The Fabrication of Gelatin–Elastin–Nanocellulose Composite Bioscaffold as a Potential Acellular Skin Substitute. Polymers 2023, 15, 779. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.-S.; Bae, H. Nanogels Derived from Fish Gelatin: Application to Drug Delivery System. Mar. Drugs 2019, 17, 246. [Google Scholar] [CrossRef]
- Shyu, Y.-S.; Chen, G.-W.; Chiang, S.-C.; Sung, W.-C. Effect of Chitosan and Fish Gelatin Coatings on Preventing the Deterioration and Preserving the Quality of Fresh-Cut Apples. Molecules 2019, 24, 2008. [Google Scholar] [CrossRef]
- Toader, A.G.; Vlasceanu, G.M.; Serafim, A.; Banciu, A.; Ionita, M. Double-Reinforced Fish Gelatin Composite Scaffolds for Osteochondral Substitutes. Materials 2023, 16, 1815. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Brachais, C.-H.; Debeaufort, F. Impact of electron beam irradiation on fish gelatin film properties. Food Chem. 2016, 195, 11–18. [Google Scholar] [CrossRef]
- Odelli, D.; Sarigiannidou, K.; Soliani, A.; Marie, R.; Mohammadifar, M.A.; Jessen, F.; Spigno, G.; Vall-llosera, M.; de Carvalho, A.F.; Verni, M.; et al. Interaction between Fish Skin Gelatin and Pea Protein at Air-Water Interface after Ultrasound Treatment. Foods 2022, 11, 659. [Google Scholar] [CrossRef]
- Devi, A.F.; Buckow, R.; Hemar, Y.; Kasapis, S. Modification of the structural and rheological properties of whey protein/gelatin mixtures through high pressure processing. Food Chem. 2014, 156, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, L.; Nie, Y.; Yang, M.; Wu, W.; Wang, Z.; Wang, X.; Zhong, J. Effect of transglutaminase crosslinking on the structural, physicochemical, functional, and emulsion stabilization properties of three types of gelatins. LWT-Food Sci. Technol. 2022, 163, 113543. [Google Scholar] [CrossRef]
- Nie, K.; Han, S.; Yang, J.; Sun, Q.; Wang, X.; Li, X.; Li, Q. Enzyme-Crosslinked Electrospun Fibrous Gelatin Hydrogel for Potential Soft Tissue Engineering. Polymers 2020, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef] [PubMed]
- Masutani, E.M.; Kinoshita, C.K.; Tanaka, T.T.; Ellison, A.K.; Yoza, B.A. Increasing thermal stability of gelatin by UV-induced cross-linking with glucose. Int. J. Biomater. 2014, 2014, 979636. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, C.; Jia, H.; Mráz, J.; Zhao, R.; Li, S.; Dong, X.; Pan, J. Modified Structural and Functional Properties of Fish Gelatin by Glycosylation with Galacto-Oligosaccharides. Foods 2023, 12, 2828. [Google Scholar] [CrossRef] [PubMed]
- Derkach, S.R.; Kolotova, D.S.; Voron’ko, N.G.; Obluchinskaya, E.D.; Malkin, A.Y. Rheological Properties of Fish Gelatin Modified with Sodium Alginate. Polymers 2021, 13, 743. [Google Scholar] [CrossRef]
- Shi, X.-D.; Huang, J.-J.; Wu, J.-L.; Cai, X.-X.; Tian, Y.-Q.; Rao, P.-F.; Huang, J.-L.; Wang, S.-Y. Fabrication, interaction mechanism, functional properties, and applications of fish gelatin-polysaccharide composites: A review. Food Hydrocoll. 2022, 122, 107106. [Google Scholar] [CrossRef]
- Azizah, F.; Nursakti, H.; Ningrum, A.; Supriyadi. Development of Edible Composite Film from Fish Gelatin–Pectin Incorporated with Lemongrass Essential Oil and Its Application in Chicken Meat. Polymers 2023, 15, 2075. [Google Scholar] [CrossRef]
- Ji, Z.; Yu, L.; Duan, Q.; Miao, S.; Liu, H.; Shen, W.; Jin, W. Morphology and Rheology of a Cool-Gel (Protein) Blended with a Thermo-Gel (Hydroxypropyl Methylcellulose). Foods 2022, 11, 128. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, S.; Lu, W.; Chen, N.; Zhu, D.; Li, Y. Preparation and characterization of enzymatically cross-linked gelatin/cellulose nanocrystal composite hydrogels. RSC Adv. 2021, 11, 10794. [Google Scholar] [CrossRef]
- Zhuang, C.; Taoa, F.; Cui, Y. Anti-degradation gelatin films crosslinked by active ester based on cellulose. RSC Adv. 2015, 5, 52183–52193. [Google Scholar] [CrossRef]
- Padhi, J.R.; Nayak, D.; Nanda, A.; Rauta, P.R.; Ashe, S.; Nayak, B. Development of highly biocompatible Gelatin & i-Carrageenan based composite hydrogels: In depth physiochemical analysis for biomedical applications. Carbohydr. Polym. 2016, 153, 292–301. [Google Scholar] [CrossRef]
- Etxabide, A.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Sustainable Fish Gelatin Films: From Food Processing Waste to Compost. ACS Sustain. Chem. Eng. 2016, 4, 4626–4634. [Google Scholar] [CrossRef]
- Gnezdilov, O.I.; Zuev, Y.F.; Zueva, O.S.; Potarikina, K.S.; Us’yarov, O.G. Self-Diffusion of Ionic Surfactants and Counterions in Premicellar and Micellar Solutions of Sodium, Lithium and Cesium Dodecyl Sulfates as Studied by NMR-Diffusometry. Appl. Magn. Reson. 2011, 40, 91–103. [Google Scholar] [CrossRef]
- Zueva, O.S.; Kusova, A.M.; Makarova, A.O.; Turanov, A.; Iskhakova, A.; Salnikov, V.V.; Zuev, Y.F. Reciprocal Effects of Multi-Walled Carbon Nanotubes and Oppositely Charged Surfactants in Bulk Water and at Interfaces. Colloids Surf. A Physicochem. Eng. Asp. 2020, 603, 125296. [Google Scholar] [CrossRef]
- Amonpattaratkit, P.; Khunmanee, S.; Kim, D.H.; Park, H. Synthesis and Characterization of Gelatin-Based Crosslinkers for the Fabrication of Superabsorbent Hydrogels. Materials 2017, 10, 826. [Google Scholar] [CrossRef]
- Uguz, S.S.; Ozel, B.; Grunin, L.; Ozvural, E.B.; Oztop, M.H. Non-Conventional Time Domain (TD)-NMR Approaches for Food Quality: Case of Gelatin-Based Candies as a Model Food. Molecules 2022, 27, 6745. [Google Scholar] [CrossRef]
- Richardson, S.J.; Steinberg, M.P. Applications of nuclear magnetic resonance. In Water Activity: Theory and Applications to Food; Rockland, L.B., Beuchart, L.R., Eds.; Marcel Dekker: New York, NY, USA, 1987; pp. 235–285. [Google Scholar]
- Cornillon, P.; Salim, L.C. Characterization of water mobility and distribution in low-and intermediate-moisture food systems. Magn. Reson. Imaging 2000, 18, 335–341. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Tashiro, Y.; Matsukava, S.; Ogava, H. Gelation Mechanism of Surimi Studied by 1H NMR Relaxation Measurements. J. Food Sci. 2007, 72, E362–E367. [Google Scholar] [CrossRef]
- McConville, P.; Whittaker, M.K.; Pope, J.M. Water and Polymer Mobility in Hydrogel Biomaterials Quantified by 1HNMR: A Simple Model Describing Both T1 and T2 Relaxation. Macromolecules 2002, 35, 6961–6969. [Google Scholar] [CrossRef]
- Kusova, A.M.; Safieva, R.Z.; Gnezdilov, O.I.; Zueva, O.S.; Salnikov, V.V.; Zuev, Y.F. Nanopore confinement and fluid behavior in nanocellulose–based hydro- and organogels. Carbohydr. Polym. Technol. Appl. 2021, 2, 100111. [Google Scholar] [CrossRef]
- McConville, P.; Pope, J.M. 1H NMR T2 relaxation in contact lens hydrogels as a probe of water mobility. Polymer 2001, 42, 3559–3568. [Google Scholar] [CrossRef]
- Barbieri, R.; Quaglia, M.; Delfini, M.; Brosio, E. Investigation of water dynamic behaviour in poly(HEMA) and poly(HEMA-co-DHPMA) hydrogels by proton T2 relaxation time and self-diffusion coefficient N.M.R. measurements. Polymer 1998, 39, 1059–1066. [Google Scholar] [CrossRef]
- Beilinson, Y.; Rassabina, A.; Lunev, I.; Faizullin, D.; Greenbaum, A.; Salnikov, V.; Zuev, Y.; Minibayeva, F.; Feldman, Y. The dielectric response of hydrated water as a structural signature of nanoconfined lichen melanins. Phys. Chem. Chem. Phys. 2022, 24, 22624–22633. [Google Scholar] [CrossRef]
- Lukichev, A.A. Nonlinear relaxation functions. Physical meaning of the Jonscher’s power law. J. Non-Cryst. Solids 2016, 442, 17–21. [Google Scholar] [CrossRef]
- Iwamoto, S.; Kumagai, H. Analysis of Dielectric Relaxation of a Gelatin Solution. Biosci. Biotechnol. Biochem. 1998, 62, 1381–1387. [Google Scholar] [CrossRef]
- Kumagai, H.; Sugiyama, T.; Iwamoto, S. Effect of Water Content on Dielectric Relaxation of Gelatin in a Glassy State. J. Agric. Food Chem. 2000, 48, 2260–2265. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Xu, Y.; Ma, Y.; Guo, X. The Structural and Functional Differences between Three Species of Fish Scale Gelatin and Pigskin Gelatin. Foods 2022, 11, 3960. [Google Scholar] [CrossRef]
- Dimitriou, C.J.; Ewolt, R.H.; McKinley, G.H. Describing and prescribing the constitutive response of yield stress fluids using large amplitude oscillatory shear stress (LAOStress). J. Rheol. 2013, 57, 27–70. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Isayev, A.I. Rheology. Concepts, Methods, and Applications, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2017. [Google Scholar]
- Kavanagh, G.M.; Ross-Murphy, S.B. Rheological characterization of polymer gels. Prog. Polym. Sci. 1998, 23, 533–562. [Google Scholar] [CrossRef]
- Clark, A.H.; Ross-Murphy, S.B. Biopolymer Network Assembly: Measurements and Theory. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: London, UK, 2009; pp. 1–27. [Google Scholar]
- Nijenhuis, K. Thermoreversible network. Adv. Polym. Sci. 1997, 130, 1–235. [Google Scholar]
- Almdal, K.; Dyre, J.; Hvidt, S.; Kramer, O. Towards a Phenomenological Definition of the Term ‘Gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Derkach, S.R.; Kulichikhin, V.G. Rheology of Gels and Yielding Liquids. Gels 2023, 9, 715. [Google Scholar] [CrossRef]
- Veis, A. The Macromolecular Chemistry of Gelatin; Academic Press: New York, NY, USA; London, UK, 1964. [Google Scholar]
- Hermida-Merino, C.; Cabaleiro, D.; Lugo, L.; Valcarcel, J.; Vázquez, J.A.; Bravo, I.; Longo, A.; Salloum-Abou-Jaoude, G.; Solano, E.; Gracia-Fernández, C.; et al. Characterization of Tuna Gelatin-Based Hydrogels as a Matrix for Drug Delivery. Gels 2022, 8, 237. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A.; Kolotova, D.S.; Gordeeva, A.M.; Faizullin, D.A.; Gusev, Y.A.; Zuev, Y.F.; Makshakova, O.N. Molecular structure and properties of κ-carrageenan-gelatin gels. Carbohydr. Polym. 2018, 197, 66–74. [Google Scholar] [CrossRef]
- Zueva, O.S.; Gubaidullin, A.T.; Makarova, A.O.; Bogdanova, L.R.; Zakharova, L.Y.; Zuev, Y.F. Structural features of composite protein-polysaccharide hydrogel in the presence of a carbon nanomaterial. Russ. Chem. Bull. 2020, 69, 581–589. [Google Scholar] [CrossRef]
- Gubaidullin, A.T.; Makarova, A.O.; Derkach, S.R.; Voron’ko, N.G.; Kadyirov, A.I.; Ziganshina, S.A.; Salnikov, V.V.; Zueva, O.S.; Zuev, Y.F. Modulation of Molecular Structure and Mechanical Properties of k-Carrageenan-Gelatin Hydrogel with Multi-Walled Carbon Nanotubes. Polymers 2022, 14, 2346. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Kadyirov, A.I.; Ziganshina, S.A.; Kazantseva, M.A.; Zueva, O.S.; Gubaidullin, A.T.; Zuev, Y.F. Supramolecular Structure and Mechanical Performance of k-Carrageenan–Gelatin Gel. Polymers 2022, 14, 4347. [Google Scholar] [CrossRef]
- Makarova, A.O.; Derkach, S.R.; Khair, T.; Kazantseva, M.A.; Zuev, Y.F.; Zueva, O.S. Ion-Induced Polysaccharide Gelation: Peculiarities of Alginate Egg-Box Association with Different Divalent Cations. Polymers 2023, 15, 1243. [Google Scholar] [CrossRef] [PubMed]
- Zueva, O.S.; Khair, T.; Derkach, S.R.; Kazantseva, M.A.; Zuev, Y.F. Strontium-Induced Gelation of Sodium Alginate in the Presence of Carbon Nanotubes: Elemental Analysis and Gel Structure. J. Compos. Sci. 2023, 7, 286. [Google Scholar] [CrossRef]
- Zueva, O.S.; Khair, T.; Kazantseva, M.A.; Latypova, L.; Zuev, Y.F. Ions-Induced Alginate Gelation According to Elemental Analysis and a Combinatorial Approach. Int. J. Mol. Sci. 2023, 24, 16201. [Google Scholar] [CrossRef]
- Ge, S.; Li, M.; Ji, N.; Liu, J.; Mul, H.; Xiong, L.; Sun, Q. Preparation of a Strong Gelatin–Short Linear Glucan Nanocomposite Hydrogel by an in Situ Self-Assembly Process. J. Agric. Food Chem. 2018, 66, 177–186. [Google Scholar] [CrossRef]
- DIFFRAC Plus Evaluation package EVA, Version 11. User’s Manual. Bruker AXS: Karlsruhe, Germany, 2005; 258p.
- Axelrod, N.; Axelrod, E.; Gutina, A.; Puzenko, A.; Ishai, B.P.; Feldman, Y. Dielectric spectroscopy data treatment: I. Frequency domain. Meas. Sci. Technol. 2004, 15, 755. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuev, Y.F.; Derkach, S.R.; Bogdanova, L.R.; Voron’ko, N.G.; Kuchina, Y.A.; Gubaidullin, A.T.; Lunev, I.V.; Gnezdilov, O.I.; Sedov, I.A.; Larionov, R.A.; et al. Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel. Gels 2023, 9, 990. https://doi.org/10.3390/gels9120990
Zuev YF, Derkach SR, Bogdanova LR, Voron’ko NG, Kuchina YA, Gubaidullin AT, Lunev IV, Gnezdilov OI, Sedov IA, Larionov RA, et al. Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel. Gels. 2023; 9(12):990. https://doi.org/10.3390/gels9120990
Chicago/Turabian StyleZuev, Yuriy F., Svetlana R. Derkach, Liliya R. Bogdanova, Nikolai G. Voron’ko, Yulia A. Kuchina, Aidar T. Gubaidullin, Ivan V. Lunev, Oleg I. Gnezdilov, Igor A. Sedov, Radik A. Larionov, and et al. 2023. "Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel" Gels 9, no. 12: 990. https://doi.org/10.3390/gels9120990
APA StyleZuev, Y. F., Derkach, S. R., Bogdanova, L. R., Voron’ko, N. G., Kuchina, Y. A., Gubaidullin, A. T., Lunev, I. V., Gnezdilov, O. I., Sedov, I. A., Larionov, R. A., Latypova, L., & Zueva, O. S. (2023). Underused Marine Resources: Sudden Properties of Cod Skin Gelatin Gel. Gels, 9(12), 990. https://doi.org/10.3390/gels9120990