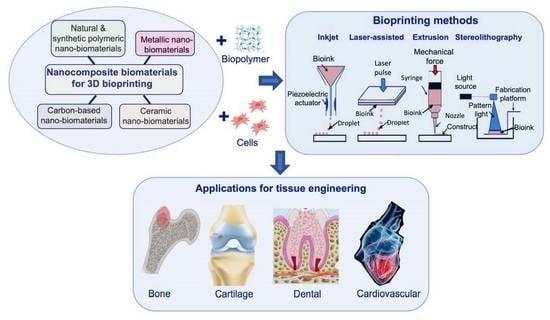
Nanocomposite Bioprinting for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials as Nanocomposites for Printing and Bioprinting
2.1. Ceramics
2.2. Carbon-Based Nanoparticles
2.3. Polymeric Nanoparticles
2.4. Metallic Nanoparticles
3. Three-Dimensional Bioprinting Techniques
3.1. Extrusion-Based 3D Bioprinting
3.2. Inkjet 3D Bioprinting
3.3. Stereolithography 3D Bioprinting
3.4. Laser-Assisted 3D Bioprinting
4. Nanocomposite Bioprinting Applications
4.1. Conventional Bioink Role and Properties for Bone and Cartilage Composite Bioprinting
4.2. Nanomaterial Composites for Bone Bioprinting
4.3. Nanomaterial Composites for Cartilage Bioprinting
4.4. Three-Dimensional Printing of Bone and Cartilage Constructs with Extracellular Matrix Composites
| Nanoparticle Additive | Bioink Composition | Cell Type | Application | Material Enhancements | References |
|---|---|---|---|---|---|
| Silica nanoparticles Calcium-functionalized Copper-functionalized Positively/negatively charged | Gelatin methacrylate Gelatin–alginate Collagen methacrylate | Bone marrow mesenchymal stem cells Human umbilical endothelial cells | Bone | Enhanced alkaline phosphatase cell activity, calcium deposition, and yield stress value. | [147,148,153,155] |
| Layered hydroxide nanoparticles | Gelatin methacrylate | N/A | Cartilage | Enhanced compressive strength, cell spreading, and cell aspect ratio. | [156] |
| Piezoelectric barium titanate nanoparticles + graphene oxide nanoflakes | Bioinstructive matrix (RGD-VitroGel) | Adipose-derived mesenchymal stem cells | Cartilage | Enhanced antibacterial effects and chondrogenic cell activity. | [157] |
| Calcium-modified silicate nanoparticles | Gelatin–alginate- Poly(ϵ-caprolactone) | Wharton’s jelly mesenchymal stem cells Human umbilical vein endothelial cells | Dental tissue | Increased Young’s modulus, angiogenic biomarkers, and bone formation proteins. | [177] |
| Zirconia nanoparticles | Acrylate ester-based dental resin | N/A | Dental tissue | Increased ductility and flexural strength and enhanced sorption in vitro. | [178] |
| Silica nanoparticles | Polymer-infiltrated ceramic network | N/A | Dental tissue | Increased shear bond strength and surface free energy. | [179] |
| Carbon nanotubes | UV collagen methacrylate and alginate | Human coronary artery endothelial cells | Cardiovascular tissue | Enhanced mechanical and electrical properties, increased stiffness, and cell attachment. | [13] |

4.5. Nanocomposites for 3D Printing and Bioprinting in Dental Tissue Engineering Applications
4.6. Bioprinting of Nanocomposites for Cardiovascular Applications
4.7. Advances of Nanocomposite Bioprinting for Organ-on-a-Chip and Biosensor Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, X.; Shi, G.; Fan, B.; Cheng, X.; Zhang, X.; Wang, X.; Liu, S.; Hao, Y.; Wei, Z.; Wang, L.; et al. Polycaprolactone Electrospun Fiber Scaffold Loaded With iPSCs-NSCs And ASCs As A Novel Tissue Engineering Scaffold For The Treatment Of Spinal Cord Injury. Int. J. Nanomed. 2019, 14, 7681–7682. [Google Scholar]
- Ressler, A. Chitosan-Based Biomaterials for Bone Tissue Engineering Applications: A Short Review. Polymers 2022, 14, 3430. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Do, A.-V.; Khorsand, B.; Geary, S.; Salem, A. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthc. Mater. 2015, 4, 1742–1762. [Google Scholar]
- Fielding, G.; Bandyopadhyay, A.; Bose, S. Effects of Silica and Zinc Oxide Doping on Mechanical and Biological Properties of 3D Printed Tricalcium Phosphate Tissue Engineering Scaffolds. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2011, 28, 113–122. [Google Scholar]
- Ronca, A.; Ambrosio, L.; Grijpma, D. Preparation of designed poly(D,L-lactide)/nano-hydroxyapatite composite structures by stereolithography. Acta Biomater. 2012, 9, 5989–5996. [Google Scholar] [CrossRef]
- Sharma, N.; Cao, S.; Msallem, B.; Kunz, C.; Brantner, P.; Honigmann, P.; Thieringer, F. Effects of Steam Sterilization on 3D Printed Biocompatible Resin Materials for Surgical Guides-An Accuracy Assessment Study. J. Clin. Med. 2020, 9, 1506. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. Effects of different sterilization processes on the properties of a novel 3D-printed polycaprolactone stent. Polym. Adv. Technol. 2018, 29, 2327–2335. [Google Scholar]
- Urbanczyk, M.; Layland, S.; Schenke-Layland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix Biol. 2019, 85–86, 1–14. [Google Scholar]
- Daley, W.; Peters, S.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Gouma, P. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2006, 2, 37–41. [Google Scholar] [CrossRef]
- Izadifar, M.; Chapman, D.; Babyn, P.; Chen, X.; Kelly, M. UV-Assisted 3D Bioprinting of Nanoreinforced Hybrid Cardiac Patch for Myocardial Tissue Engineering. Tissue Eng. Part C Methods 2018, 24, 74–88. [Google Scholar]
- Antich Acedo, C.; de Vicente, J.; Jiménez González, G.; Chocarro, C.; Carrillo, E.; Montañez, E.; Gálvez, P.; Marchal, J. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020, 106, 114–123. [Google Scholar] [CrossRef]
- Bedell, M.; Navara, A.; Du, Y.; Zhang, S.; Mikos, A. Polymeric Systems for Bioprinting. Chem. Rev. 2020, 120, 10744–10792. [Google Scholar] [CrossRef]
- Reid, J.; Mollica, P.; Johnson, G.; Ogle, R.; Bruno, R.; Sachs, P. Accessible bioprinting: Adaptation of a low-cost 3D-printer for precise cell placement and stem cell differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Wonwoo, J.; Lee, S.; Kim, J.B.; Jin, S.; Kang, H.-W. Decellularized extracellular matrix-based bio-ink with enhanced 3D printability and mechanical properties. Biofabrication 2019, 12, 025003. [Google Scholar] [CrossRef]
- Narayanan, L.K.; Huebner, P.; Fisher, M.; Spang, J.; Starly, B.; Shirwaiker, R. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber-Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells. ACS Biomater. Sci. Eng. 2016, 2, 1732–1742. [Google Scholar]
- Ng, W.L.; Yeong, W.Y.; Win Naing, M. Polyvinylpyrrolidone-Based Bio-Ink Improves Cell Viability and Homogeneity during Drop-On-Demand Printing. Materials 2017, 10, 190. [Google Scholar] [CrossRef]
- Chang, R.; Nam, J.; Sun, W. Effects of Dispensing Pressure and Nozzle Diameter on Cell Survival from Solid Freeform Fabrication–Based Direct Cell Writing. Tissue Eng. Part A 2008, 14, 41–48. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.; Shiwarski, D.; Tashman, J.; Hinton, T.; Yerneni, S.; Bliley, J.; Campbell, P.; Feinberg, A. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Gao, G.; Schilling, A.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar]
- Solis, L.; Ayala, Y.; Portillo, S.; Varela-Ramirez, A.; Aguilera, R.; Boland, T. Thermal inkjet bioprinting triggers the activation of the VEGF pathway in human microvascular endothelial cells in vitro. Biofabrication 2019, 11, 045005. [Google Scholar] [PubMed]
- Elomaa, L.; Pan, C.-C.; Shanjani, Y.; Malkovskiy, A.; Seppälä, J.V.; Yang, Y. Three-dimensional fabrication of cell-laden biodegradable poly(ethylene glycol-co-depsipeptide) hydrogels by visible light stereolithography. J. Mater. Chem. B 2015, 3, 8348–8358. [Google Scholar] [PubMed] [Green Version]
- Thomas, A.; Orellano, I.; Lam, T.; Noichl, B.; Geiger, M.-A.; Amler, A.-K.; Kreuder, A.-E.; Palmer, C.; Duda, G.; Lauster, R.; et al. Vascular bioprinting with enzymatically degradable bioinks via multi-material projection-based stereolithography. Acta Biomater. 2020, 117, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Souquet, A.; Catros, S.; Duocastella, M.; Pippenger, B.; Bellance, S.; Bareille, R.; Rémy, M.; Bordenave, L.; Guillemot, F. Laser assisted bioprinting of engineered tissue with high cell density and microscale organization. Biomaterials 2010, 31, 7250–7256. [Google Scholar] [PubMed]
- Keriquel, V.; Oliveira, H.; Rémy, M.; Ziane, S.; Delmond, S.; Rousseau, B.; Rey, S.; Catros, S.; Amédée, J.; Guillemot, F.; et al. In situ printing of mesenchymal stromal cells, by laser-assisted bioprinting, for in vivo bone regeneration applications. Sci. Rep. 2017, 7, 1778. [Google Scholar] [CrossRef] [Green Version]
- Kacarevic, Z.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanisevic, Z.; Barbeck, M. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials 2018, 11, 2199. [Google Scholar]
- Ramadan, Q.; Zourob, M. 3D Bioprinting at the Frontier of Regenerative Medicine, Pharmaceutical, and Food Industries. Front. Med. Technol. 2020, 2, 607648. [Google Scholar]
- Zhiwei, J.; Bin, L.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D printing of HA / PCL composite tissue engineering scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthes, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.-z. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar] [CrossRef] [Green Version]
- Soenen, S.; Parak, W.; Rejman, J.; Manshian, B. (Intra)Cellular Stability of Inorganic Nanoparticles: Effects on Cytotoxicity, Particle Functionality, and Biomedical Applications. Chem. Rev. 2015, 115, 150202072049004. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.; Quinnell, S.; Wei, M. Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds. J. Biomed. Mater. Res. Part A 2017, 105, 1457–1468. [Google Scholar]
- Kosik-Kozioł, A.; Costantini, M.; Mróz, A.; Idaszek, J.; Heljak, M.; Jaroszewicz, J.; Kijeńska-Gawrońska, E.; Szoke, K.; Frerker, N.; Barbetta, A.; et al. 3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering. Biofabrication 2019, 11, 035016. [Google Scholar]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.; Losic, D. 3D Bioprinting of Cell-Laden Electroconductive MXene Nanocomposite Bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.; Prasai, D.; Rath, R.; Balikov, D.; Bae, H.; Bolotin, K.; Sung, H.-J. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 2013, 5, 4171–4176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Hudson, J.; Spicer, P.; Tour, J.; Krishnamoorti, R.; Mikos, A. Injectable Nanocomposites of Single-Walled Carbon Nanotubes and Biodegradable Polymers for Bone Tissue Engineering. Biomacromolecules 2006, 7, 2237–2242. [Google Scholar] [PubMed]
- Serafin, A.; Murphy, C.; Culebras, M.; Collins, M. Printable Alginate/Gelatine Hydrogel Reinforced With Carbon Nanofibers As Electrically Conductive Scaffolds For Tissue Engineering. Mater. Sci. Eng. C 2021, 122, 111927. [Google Scholar] [CrossRef]
- Wilson, S.; Cross, L.; Peak, C.; Gaharwar, A. Shear-Thinning and Thermo-Reversible Nanoengineered Inks for 3D Bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar]
- Erisken, C.; Kalyon, D.; Wang, H. Functionally Graded Electrospun Polycaprolactone and b-Tricalcium Phosphate Nanocomposites for Tissue Engineering Applications. Biomaterials 2008, 29, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2019, 103, 109731. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Zhu, M.; Zhang, Y.; Liu, Z.; Tao, C.; Zhu, Y.; Zhang, C. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015, 12, 270–280. [Google Scholar] [PubMed]
- Shrestha, B.K.; Shrestha, S.; Tiwari, A.P.; Kim, J.-I.; Ko, S.W.; Kim, H.-J.; Park, C.H.; Kim, C.S. Bio-inspired hybrid scaffold of zinc oxide-functionalized multi-wall carbon nanotubes reinforced polyurethane nanofibers for bone tissue engineering. Mater. Des. 2017, 133, 69–81. [Google Scholar]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D printing of nano-cellulosic biomaterials for medical applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar]
- Wei, P.; Xu, Y.; Gu, Y.; Yao, Q.; Li, J.; Wang, L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020, 27, 1106–1114. [Google Scholar]
- Diniz, F.R.; Maia, R.C.A.P.; de Andrade, L.R.M.; Andrade, L.N.; Vinicius Chaud, M.; da Silva, C.F.; Corrêa, C.B.; de Albuquerque Junior, R.L.C.; Pereira da Costa, L.; Shin, S.R.; et al. Silver Nanoparticles-Composing Alginate/Gelatine Hydrogel Improves Wound Healing In Vivo. Nanomaterials 2022, 12, 4071. [Google Scholar] [CrossRef]
- Saraiva, A.S.; Ribeiro, I.A.C.; Fernandes, M.H.; Cerdeira, A.C.; Vieira, B.J.C.; Waerenborgh, J.C.; Pereira, L.C.J.; Cláudio, R.; Carmezim, M.J.; Gomes, P.; et al. 3D-printed platform multi-loaded with bioactive, magnetic nanoparticles and an antibiotic for re-growing bone tissue. Int. J. Pharm. 2021, 593, 120097. [Google Scholar]
- del Pino, P.; Yang, F.; Pelaz, B.; Zhang, Q.; Kantner, K.; Hartmann, R.; Martinez de Baroja, N.; Gallego, M.; Möller, M.; Manshian, B.B.; et al. Basic Physicochemical Properties of Polyethylene Glycol Coated Gold Nanoparticles that Determine Their Interaction with Cells. Angew. Chem. Int. Ed. 2016, 55, 5483–5487. [Google Scholar]
- Manita, P.G.; Garcia-Orue, I.; Santos-Vizcaíno, E.; Hernández, R.M.; Igartua, M. 3D Bioprinting of Functional Skin Substitutes: From Current Achievements to Future Goals. Pharmaceuticals 2021, 14, 362. [Google Scholar] [CrossRef]
- Shiwarski, D.; Hudson, A.; Tashman, J.; Feinberg, A. Emergence of FRESH 3D printing as a platform for advanced tissue biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef]
- Navara, A.M.; Kim, Y.S.; Xu, Y.; Crafton, C.L.; Diba, M.; Guo, J.L.; Mikos, A.G. A dual-gelling poly(N-isopropylacrylamide)-based ink and thermoreversible poloxamer support bath for high-resolution bioprinting. Bioact. Mater. 2022, 14, 302–312. [Google Scholar] [PubMed]
- Ravanbakhsh, H.; Zhang, Y.S. Cryobioprinting for biomedical applications. J. 3d Print. Med. 2022, 6, 163–166. [Google Scholar] [CrossRef]
- Luo, Z.; Tang, G.; Ravanbakhsh, H.; Li, W.; Wang, M.; Kuang, X.; Garciamendez-Mijares, C.E.; Lian, L.; Yi, S.; Liao, J.; et al. Vertical Extrusion Cryo(bio)printing for Anisotropic Tissue Manufacturing. Adv. Mater. 2022, 34, 2108931. [Google Scholar]
- Bi, H.; Jia, X.; Ye, G.; Ren, Z.; Yang, H.; Guo, R.; Xu, M.; Cai, L.; Huang, Z. Three-Dimensional-Printed Shape Memory Biomass Composites for Thermal-Responsive Devices. 3d Print. Addit. Manuf. 2020, 7, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-W.; Liu, T.-Y.; Pang, D.-C.; Hung, J.-C.; Tseng, C.-C. Inkjet Printing of a pH Sensitive Palladium Catalyst Patterns of ITO Glass for Electroless Copper. Surf. Coat. Technol. 2014, 259, 340–345. [Google Scholar]
- Choi, K.H.; Sajid, M.; Aziz, S.; Yang, B.-S. Wide range high speed relative humidity sensor based on PEDOT:PSS–PVA composite on an IDT printed on piezoelectric substrate. Sens. Actuators A. Phys. 2015, 228, 40–49. [Google Scholar]
- Podstawczyk, D.; Nizioł, M.; Szymczyk, P.; Wiśniewski, P.; Guiseppi-Elie, A. 3D printed stimuli-responsive magnetic nanoparticle embedded alginate-methylcellulose hydrogel actuators. Addit. Manuf. 2020, 34, 101275. [Google Scholar] [CrossRef]
- Ding, Z.; Yuan, C.; Peng, X.; Wang, T.; Qi, H.; Dunn, M. Direct 4D Printing via Active Composite Materials. Sci. Adv. 2017, 3, e1602890. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-C.; Zhang, Y.S.; Akpek, A.; Shin, S.; Khademhosseini, A. 4D bioprinting: The next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication 2016, 9, 012001. [Google Scholar]
- Hassan, M.; Dave, K.; Chandrawati, R.; Dehghani, F.; Gomes, V. 3D printing of biopolymer nanocomposites for tissue engineering: Nanomaterials, processing and structure-function relation. Eur. Polym. J. 2019, 121, 109340. [Google Scholar]
- Bose, S.; Tarafder, S. Calcium Phosphate Ceramic Systems in Growth Factor and Drug Delivery for Bone Tissue Engineering: A Review. Acta Biomater. 2011, 8, 1401–1421. [Google Scholar]
- Zhang, Y.; Wang, F.; Tan, H.; Chen, G.; Guo, L.; Yang, L. Analysis of the Mineral Composition of the Human Calcified Cartilage Zone. Int. J. Med. Sci. 2012, 9, 353–360. [Google Scholar]
- Kumai, T.; Yui, N.; Yatabe, K.; Sasaki, C.; Fujii, R.; Takenaga, M.; Fujiya, H.; Niki, H.; Yudoh, K. A novel, self-assembled artificial cartilage– hydroxyapatite conjugate for combined articular cartilage and subchondral bone repair: Histopathological analysis of cartilage tissue engineering in rat knee joints. Int. J. Nanomed. 2019, 14, 1283–1298. [Google Scholar]
- Catros, S.; Fricain, j.c.; Guillotin, B.; Pippenger, B.; Bareille, R.; Remy, M.; Lebraud, E.; Desbat, B.; Guillemot, F. Laser-Assisted Bioprinting for Creating on-Demand Patterns of Human Osteoprogenitor Cells and Nano-Hydroxyapatite. Biofabrication 2011, 3, 025001. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Eberhardt, C.; Pagliccia, G.; Kenkel, D.; Grande, D.; Boss, A.; Zenobi-Wong, M. Bioprinting Complex Cartilaginous Structures with Clinically Compliant Biomaterials. Adv. Funct. Mater. 2015, 25, 7406–7417. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, A.; Janarthanan, G.; Tran, H.; Ham, H.; Yoon, J.; Noh, I. Bioink homogeneity control during 3D bioprinting of multicomponent micro/nanocomposite hydrogel for even tissue regeneration using novel twin screw extrusion system. Chem. Eng. J. 2021, 415, 128971. [Google Scholar]
- Yao, J.; Radin, S.; Leboy, P.; Ducheyne, P. The effect of bioactive glass content on synthesis and bioactivity of composite poly (lactic-co-glycolic acid)/bioactive glass substrate for tissue engineering. Biomaterials 2005, 26, 1935–1943. [Google Scholar] [CrossRef]
- Pereira, M.; Jones, J.; Hench, L.L. Bioactive Glass and Hybrid Scaffolds Prepared by Sol-Gel Method for Bone Tissue Engineering. Adv. Appl. Ceram. 2005, 104, 35–42. [Google Scholar] [CrossRef]
- Wang, X.; Tolba, E.; Schröder, H.C.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Müller, W. Effect of Bioglass on Growth and Biomineralization of SaOS-2 Cells in Hydrogel After 3D Cell Bioprinting. PLoS ONE 2014, 9, e112497. [Google Scholar]
- Guduric, V.; Wieckhusen, J.; Bernhardt, A.; Ahlfeld, T.; Lode, A.; Wu, C.; Gelinsky, M. Composite Bioinks With Mesoporous Bioactive Glasses-A Critical Evaluation of Results Obtained by In Vitro Experiments. Front. Bioeng. Biotechnol. 2021, 9, 767256. [Google Scholar] [CrossRef] [PubMed]
- Kolan, K.; Semon, J.; Bindbeutel, A.; Day, D.; Leu, M. Bioprinting with bioactive glass loaded polylactic acid composite and human adipose stem cells. Bioprinting 2020, 18, e00075. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Semon, J.A.; Bromet, B.; Day, D.E.; Leu, M.C. Bioprinting with human stem cell-laden alginate-gelatin bioink and bioactive glass for tissue engineering. Int. J. Bioprinting 2019, 5, 204. [Google Scholar]
- Murphy, C.; Kolan, K.; Li, W.; Semon, J.; Day, D.; Leu, M. 3D bioprinting of stem cells and polymer/bioactive glass composite scaffolds for bone tissue engineering. Int. J. Bioprinting 2017, 3, 5. [Google Scholar]
- Tomás, H.; Alves, C.; Rodrigues, J. Laponite®: A key nanoplatform for biomedical applications? Nanomed. Nanotechnol. Biol. Med. 2017, 14, 2407–2420. [Google Scholar]
- Gonzaga, V.; Lima Poli, A.; Gabriel, J.; Tezuka, D.; Valdes, T.; Leitao, A.; Rodero, C.; Bauab, T.; Chorilli, M.; Schmitt Cavalheiro, C. Chitosan-laponite nanocomposite scaffolds for wound dressing application. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2020, 108, 1388–1397. [Google Scholar]
- Miao, S.; Zhou, J.; Liu, B.; Lei, X.; Wang, T.; Hao, X.; Cheng, P.; Wu, H.; Song, Y.; Pei, G.; et al. A 3D bioprinted nano-laponite hydrogel construct promotes osteogenesis by activating PI3K/AKT signaling pathway. Materials Today Bio. 2022, 16, 100342. [Google Scholar]
- Ma, Z.; He, H.; Deng, C.; Ren, Y.; Lu, D.; Li, W.; Sun, X.; Wang, W.; Zhang, Y.; Xu, Y.; et al. 3D bioprinting of proangiogenic constructs with induced immunomodulatory microenvironments through a dual cross-linking procedure using laponite incorporated bioink. Compos. Part B Eng. 2022, 229, 109399. [Google Scholar] [CrossRef]
- Nadernezhad, A.; Caliskan, O.; Topuz, F.; Afghah, F.; Erman, B.; Koc, B. Nanocomposite Bioinks Based on Agarose and 2D Nanosilicates with Tunable Flow Properties and Bioactivity for 3D Bioprinting. ACS Appl. Bio Mater. 2019, 2, 796–806. [Google Scholar] [CrossRef]
- Cidonio, G.; Alcala, C.; Lim, K.; Glinka, M.; Mutreja, I.; Kim, Y.-H.; Dawson, J.; Woodfield, T.; Oreffo, R. Osteogenic and angiogenic tissue formation in high fidelity nanocomposite Laponite-gelatin bioinks. Biofabrication 2019, 11, 035027. [Google Scholar]
- Jiang, J.-W.; Wang, B.-S.; Wang, J.-S.; Park, H. A Review on Flexural Mode of Graphene: Lattice Dynamics, Thermal Conduction, Thermal Expansion, Elasticity, and Nanomechanical Resonance. J. Phys. Condens. Matter Inst. Phys. J. 2014, 27, 083001. [Google Scholar]
- Randviir, E.P.; Brownson, D.A.C.; Banks, C.E. A decade of graphene research: Production, applications and outlook. Mater. Today 2014, 17, 426–432. [Google Scholar] [CrossRef]
- Smith, A.; Lachance, A.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene, graphene oxide and other graphenoids: Review. Electrochem. Commun. 2013, 36, 14–18. [Google Scholar] [CrossRef]
- Shamekhi, M.A.; Mirzadeh, H.; Mahdavi, H.; Rabiee, A.; Mohebbi-Kalhori, D.; Baghaban Eslaminejad, M. Graphene oxide containing chitosan scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2019, 127, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Dinescu, S.; Ionita, M.; Pandele, A.; Galateanu, B.; Iovu, H.; Ardelean, A.; Costache, M.; Hermenean, A. In vitro cytocompatibility evaluation of chitosan/graphene oxide 3D scaffold composites designed for bone tissue engineering. Bio-Med. Mater. Eng. 2014, 24, 2249–2256. [Google Scholar]
- Zhang, J.; Eyisoylu, H.; Qin, X.-H.; Rubert, M.; Müller, R. 3d Bioprinting of Graphene Oxide-Incorporated Cell-Laden Bone Mimicking Scaffolds for Promoting Scaffold Fidelity, Osteogenic Differentiation and Mineralization. SSRN Electron. J. 2020, 121, 637–652. [Google Scholar] [CrossRef]
- Zhu, W.; Harris, M.D.P.B.T.; Zhang, L. Gelatin Methacrylamide Hydrogel with Graphene Nanoplatelets for Neural Cell-Laden 3D Bioprinting. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2016), Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 4185–4188. [Google Scholar]
- Choe, G.; Oh, S.; Seok, J.M.; Park, S.; Lee, J. Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications. Nanoscale 2019, 11, 23275–23285. [Google Scholar]
- Huang, C.-T.; Shrestha, L.; Ariga, K.; Hsu, S.-h. A graphene–polyurethane composite hydrogel as a potential bioink for 3D bioprinting and differentiation of neural stem cells. J. Mater. Chem. B 2017, 5, 8854–8864. [Google Scholar]
- Tran, P.; Zhang, L.; Webster, T. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar]
- Samadian, H.; Mobasheri, H.; Hasanpour Tadi, S.; Ai, J.; Azamie, M.; Faridi-Majidi, R. Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering. J. Mol. Liq. 2019, 298, 112021. [Google Scholar] [CrossRef]
- Flores-Cedillo, M.; Alvarado Estrada, K.; Pozos-Guillen, A.; Murguia, J.; Vidal, M.; Cervantes, M.; Rosales-Ibanez, R.; Cauich, J. Multiwall carbon nanotubes/polycaprolactone scaffolds seeded with human dental pulp stem cells for bone tissue regeneration. J. Mater. Sci. Mater. Med. 2015, 27, 35. [Google Scholar] [PubMed]
- Mahmoud, A.; Elbackly, R.; Taha, N.; El- Maghraby, A.; Kandil, S. Preparation and characterization of carbon nanofibrous/hydroxyapatite sheets for bone tissue engineering. Mater. Sci. Eng. C 2017, 76, 1188–1195. [Google Scholar]
- Chinga-Carrasco, G. Potential and Limitations of Nanocelluloses as Components in Biocomposite Inks for Three-Dimensional Bioprinting and for Biomedical Devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [PubMed]
- Xu, C.; Zhang Molino, B.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar]
- Rees, A.; Powell, L.; Chinga Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.; Thomas, D. 3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing Applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez Ávila, H.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi, M. Alginate Sulfate–Nanocellulose Bioinks for Cartilage Bioprinting Applications. Ann. Biomed. Eng. 2016, 45, 210–223. [Google Scholar]
- Cernencu, A.I.; Lungu, A.; Dragusin, D.M.; Stancu, I.C.; Dinescu, S.; Balahura, L.R.; Mereuta, P.; Costache, M.; Iovu, H. 3D Bioprinting of Biosynthetic Nanocellulose-Filled GelMA Inks Highly Reliable for Soft Tissue-Oriented Constructs. Materials 2021, 14, 4891. [Google Scholar]
- Nguyen, D.; Hägg, D.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [Green Version]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Maturavongsadit, P.; Narayanan, L.; Chansoria, P.; Shirwaiker, R.; Benhabbour, S. Cell-Laden Nanocellulose/Chitosan-Based Bioinks for 3D Bioprinting and Enhanced Osteogenic Cell Differentiation. ACS Appl. Bio Mater. 2021, 4, 2342–2353. [Google Scholar] [CrossRef] [PubMed]
- Kayser, L.; Lipomi, D. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Z.; Ouyang, J. Thermoelectric Properties of PEDOT:PSS. Adv. Electron. Mater. 2019, 5, 1800769. [Google Scholar]
- Babaie, A.; Bakhshandeh, B.; Abedi, A.; Mohammadnejad, J.; Shabani, I.; Ardeshirylajimi, A.; Moosavi, S.; Amini, J.; Tayebi, L. Synergistic Effects of Conductive PVA/PEDOT Electrospun Scaffolds and Electrical Stimulation for More Effective Neural Tissue Engineering. Eur. Polym. J. 2020, 140, 110051. [Google Scholar]
- Wang, S.; Guan, S.; Li, W.; Ge, D.; Xu, J.; Sun, C.; Liu, T.; Ma, X. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng. C 2018, 93, 890–901. [Google Scholar]
- Wang, Y.; Wang, Q.; Chen, Z.; Zheng, X.; Kankala, R.K.; Chen, A.-Z.; Wang, S. 3D Bioprinting of Conductive Hydrogel for Enhanced Myogenic Differentiation. Regen. Biomater. 2021, 8, rbab035. [Google Scholar]
- Roshanbinfar, K.; Vogt, L.; Greber, B.; Diecke, S.; Boccaccini, A.; Scheibel, T.; Engel, F. Electroconductive Biohybrid Hydrogel for Enhanced Maturation and Beating Properties of Engineered Cardiac Tissues. Adv. Funct. Mater. 2018, 28, 1803951. [Google Scholar]
- Gao, C.; Li, Y.; Liu, X.; Huang, J.; Zhang, Z. 3D bioprinted conductive spinal cord biomimetic scaffolds for promoting neuronal differentiation of neural stem cells and repairing of spinal cord injury. Chem. Eng. J. 2023, 451, 138788. [Google Scholar] [CrossRef]
- Spencer, A.R.; Shirzaei Sani, E.; Soucy, J.R.; Corbet, C.C.; Primbetova, A.; Koppes, R.A.; Annabi, N. Bioprinting of a Cell-Laden Conductive Hydrogel Composite. ACS Appl. Mater. Interfaces 2019, 11, 30518–30533. [Google Scholar]
- Song, S.; Liu, X.; Huang, J.; Zhang, Z. Neural stem cell-laden 3D bioprinting of polyphenol-doped electroconductive hydrogel scaffolds for enhanced neuronal differentiation. Biomater. Adv. 2022, 133, 112639. [Google Scholar] [CrossRef]
- Hasan, A.; Morshed, M.; Memic, A.; Hassan, S.; Webster, T.; Marei, H. Nanoparticles in tissue engineering: Applications, challenges and prospects. Int. J. Nanomed. 2018, 13, 5637–5655. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Shin, S.; Kempen, T.; Li, Y.-C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar]
- Ratia, C.; Soengas, R.G.; Soto, S.M. Gold-Derived Molecules as New Antimicrobial Agents. Front. Microbiol. 2022, 13, 846959. [Google Scholar]
- Gandhimathi, C.; Quek, Y.J.; Ezhilarasu, H.; Ramakrishna, S.; Bay, B.-H.; Srinivasan, D.K. Osteogenic Differentiation of Mesenchymal Stem Cells with Silica-Coated Gold Nanoparticles for Bone Tissue Engineering. Int. J. Mol. Sci. 2019, 20, 5135. [Google Scholar] [CrossRef] [Green Version]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Med. 2018, 29, 134. [Google Scholar]
- Baei, P.; Jalili, S.; Rajabi, S.; Tafazzoli-Shadpour, M.; Baharvand, H.; Aghdami, N. Electrically Conductive Gold Nanoparticle-Chitosan Thermosensitive Hydrogels for Cardiac Tissue Engineering. Mater. Sci. Eng. C 2016, 63, 131–141. [Google Scholar] [CrossRef]
- Masud, M.; Na, J.; Lin, T.-E.; Malgras, V.; Preet, A.; Sina, A.A.I.; Wood, K.; Billah, M.; Kim, J.; You, J.; et al. Nanostructured mesoporous gold biosensor for microRNA detection at attomolar level. Biosens. Bioelectron. 2020, 168, 112429. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. A Colorimetric Lead Biosensor Using DNAzyme-Directed Assembly of Gold Nanoparticles. J. Am. Chem. Soc. 2003, 125, 6642–6643. [Google Scholar] [CrossRef]
- Sekaran, S.; Nethala, S.; Pattnaik, S.; Tripathi, A.; Ambigapathi, M.; Selvamurugan, N. Preparation, characterization and antimicrobial activity of a bio-composite scaffold containing chitosan/nano-hydroxyapatite/nano-silver for bone tissue engineering. Int. J. Biol. Macromol. 2011, 49, 188–193. [Google Scholar]
- Waibhaw, G.; Hasan, A.; Jawed, A.; Pandey, L. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. j. Biol. Macromol. 2018, 111, 923–934. [Google Scholar]
- Agarwala, S.; Ng, W.L.; Lee, J.M.; Layani, M.; Yeong, W.Y.; Magdassi, S. A novel 3D bioprinted flexible and biocompatible hydrogel bioelectronic platform. Biosens. Bioelectron. 2017, 102, 365–371. [Google Scholar] [PubMed]
- Kumar, N.; Dayananda, D.; Chandran, G.; Ghosh, N.; Karthikeyan, G.; Waigaonkar, S.; Ganguly, A. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boularaoui, S.; Shanti, A.; Lanotte, M.; Luo, S.; Bawazir, S.; Lee, S.; Christoforou, N.; Khan, K.A.; Stefanini, C. Nanocomposite Conductive Bioinks Based on Low-Concentration GelMA and MXene Nanosheets/Gold Nanoparticles Providing Enhanced Printability of Functional Skeletal Muscle Tissues. ACS Biomater. Sci. Eng. 2021, 7, 5810–5822. [Google Scholar] [CrossRef]
- Alcala-Orozco, C.R.; Mutreja, I.; Cui, X.; Kumar, D.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Design and characterisation of multi-functional strontium-gelatin nanocomposite bioinks with improved print fidelity and osteogenic capacity. Bioprinting 2020, 18, e00073. [Google Scholar]
- Hermanová, S.; Pumera, M. Biodegradable polyester platform for extrusion-based bioprinting. Bioprinting 2022, 26, e00198. [Google Scholar] [CrossRef]
- Ooi, H.W.; Mota, C.; Ten Cate, A.T.; Calore, A.; Moroni, L.; Baker, M.B. Thiol–Ene Alginate Hydrogels as Versatile Bioinks for Bioprinting. Biomacromolecules 2018, 19, 3390–3400. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, D.; Anand, S.; Naing, M.W. The arrival of commercial bioprinters-Towards 3D bioprinting revolution! Int. J. Bioprinting 2018, 4, 139. [Google Scholar]
- Emmermacher, J.; Spura, D.; Cziommer, J.; Kilian, D.; Wollborn, T.; Fritsching, U.; Steingroewer, J.; Walther, T.; Gelinsky, M.; Lode, A. Engineering considerations on extrusion-based bioprinting: Interactions of material behavior, mechanical forces and cells in the printing needle. Biofabrication 2020, 12, 025022. [Google Scholar]
- Bedell, M.L.; Torres, A.L.; Hogan, K.J.; Wang, Z.; Wang, B.; Melchiorri, A.J.; Grande-Allen, K.J.; Mikos, A.G. Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting. Biofabrication 2022, 14, 045012. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar]
- Gudapati, H.; Ozbolat, I.T. The role of concentration on drop formation and breakup of collagen, fibrinogen, and thrombin solutions during inkjet bioprinting. bioRxiv 2020, 36, 15373–15385. [Google Scholar] [CrossRef]
- Dufour, A.; Gallostra, X.B.; O'Keeffe, C.; Eichholz, K.; Von Euw, S.; Garcia, O.; Kelly, D.J. Integrating melt electrowriting and inkjet bioprinting for engineering structurally organized articular cartilage. Biomaterials 2022, 283, 121405. [Google Scholar] [CrossRef]
- Kumar, H.; Kim, K. Stereolithography 3D Bioprinting; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2140, pp. 93–108. [Google Scholar]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar]
- Mandrycky, C.; Wang, D.Z.; Kim, K.; Kim, D.-H. 3D Bioprinting for Engineering Complex Tissues. Biotechnol. Adv. 2015, 34, 422–434. [Google Scholar]
- Yang, H.; Yang, K.-H.; Narayan, R.J.; Ma, S. Laser-based bioprinting for multilayer cell patterning in tissue engineering and cancer research. Essays Biochem. 2021, 65, 409–416. [Google Scholar]
- Kérourédan, O.; Bourget, J.-M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar]
- Sorkio, A.; Koch, L.; Koivusalo, L.; Deiwick, A.; Miettinen, S.; Chichkov, B.; Skottman, H. Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks. Biomaterials 2018, 171, 57–71. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, 1801048. [Google Scholar]
- Abdollahiyan, P.; Oroojalian, F.; Mokhtarzadeh, A.; de la Guardia, M. Hydrogel-Based 3D Bioprinting for Bone and Cartilage Tissue Engineering. Biotechnol. J. 2020, 15, 2000095. [Google Scholar]
- Luo, C.; Xie, R.; Zhang, J.; Liu, Y.; Li, Z.; Zhang, Y.; Zhang, X.; Yuan, T.; Chen, Y.; Fan, W. Low temperature 3D printing of tissue cartilage engineered with gelatin methacrylamide. Tissue Eng. Part C Methods 2020, 26, 306–316. [Google Scholar]
- Monavari, M.; Homaeigohar, S.; Fuentes, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112470. [Google Scholar] [CrossRef]
- Kajave, N.S.; Schmitt, T.; Nguyen, T.-U.; Gaharwar, A.K.; Kishore, V. Bioglass incorporated methacrylated collagen bioactive ink for 3D printing of bone tissue. Biomed. Mater. 2021, 16, 035003. [Google Scholar] [CrossRef]
- Gharacheh, H.; Guvendiren, M. Cell-Laden Composite Hydrogel Bioinks with Human Bone Allograft Particles to Enhance Stem Cell Osteogenesis. Polymers 2022, 14, 3788. [Google Scholar]
- Lafuente-Merchan, M.; Ruiz-Alonso, S.; García Villén, F.; Zabala, A.; Retana, A.; Gallego, I.; Saenz del Burgo, L.; Pedraz, J. 3D Bioprinted Hydroxyapatite or Graphene Oxide Containing Nanocellulose-Based Scaffolds for Bone Regeneration. Macromol. Biosci. 2022, 22, 2200236. [Google Scholar]
- Kang, M.; Kang, J.; Phuong, L.; Park, K.; Hong, S.W.; Choi, Y.S.; Han, D.-W.; Park, K. Three-Dimensional Printable Gelatin Hydrogels Incorporating Graphene Oxide to Enable Spontaneous Myogenic Differentiation. ACS Macro Lett. 2021, 10, 426–432. [Google Scholar]
- Zhu, S.; Yao, L.; Pan, C.; Tian, J.; Li, L.; Luo, B.; Zhou, C.; Lu, L. 3D printed gellan gum/graphene oxide scaffold for tumor therapy and bone reconstruction. Compos. Sci. Technol. 2021, 208, 108763. [Google Scholar] [CrossRef]
- Lee, M.; Bae, K.; Levinson, C.; Zenobi, M. Nanocomposite bioink exploits dynamic covalent bonds between nanoparticles and polysaccharides for precision bioprinting. Biofabrication 2020, 12, 025025. [Google Scholar]
- Tavares, M.T.; Gaspar, V.M.; Monteiro, M.V.; S Farinha, J.P.; Baleizão, C.; Mano, J.F. GelMA/bioactive silica nanocomposite bioinks for stem cell osteogenic differentiation. Biofabrication 2021, 13, 035012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Monavari, M.; Zheng, K.; Distler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 2022, 18, 2104996. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Lei, X.; Cheng, P.; Song, Y.; Gao, Y.; Hu, J.; Wang, C.; Zhang, S.; Li, D.; et al. 3D-bioprinted functional and biomimetic hydrogel scaffolds incorporated with nanosilicates to promote bone healing in rat calvarial defect model. Mater. Sci. Eng. C 2020, 112, 110905. [Google Scholar]
- Alarcin, E.; İzbudak, B.; Yüce Erarslan, E.; Tietilu, S.; Tutar, R.; Titi, K.; Kocaaga, B.; Guner, S.; Bal Öztürk, A. Optimization of methacrylated gelatin/layered double hydroxides nanocomposite cell-laden hydrogel bioinks with high printability for 3D extrusion bioprinting. J. Biomed. Mater. Res. Part A 2022, 111, 209–223. [Google Scholar]
- Ricotti, L.; Cafarelli, A.; Manferdini, C.; Trucco, D.; Vannozzi, L.; Gabusi, E.; Fontana, F.; Dolzani, P.; Saleh, Y.; Lenzi, E.; et al. Ultrasound stimulation of piezoelectric nanocomposite hydrogels boosts cartilage regeneration. Phys. Sci. 2022. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F. Nanocomposite scaffolds based on gelatin and alginate reinforced by Zn2SiO4 with enhanced mechanical and chemical properties for Tissue Engineering. Arab. J. Chem. 2022, 15, 103730. [Google Scholar]
- Wu, T.; Ye, J.; Zeng, K. 3D Printed Hydroxyapatite Nanocomposite Biomaterials in Orthopedic Trauma Surgery. Sci. Adv. Mater. 2021, 13, 1144–1154. [Google Scholar]
- Chakraborty, J.; Majumder, N.; Sharma, A.; Prasad, S.; Ghosh, S. 3D bioprinted silk-reinforced Alginate-Gellan Gum constructs for cartilage regeneration. Bioprinting 2022, 28, e00232. [Google Scholar]
- Bedell, M.L.; Wang, Z.; Hogan, K.J.; Torres, A.L.; Pearce, H.A.; Chim, L.K.; Grande-Allen, K.J.; Mikos, A.G. The effect of multi-material architecture on the ex vivo osteochondral integration of bioprinted constructs. Acta Biomater. 2023, 155, 99–112. [Google Scholar]
- Xing, F.; Xiang, Z.; Rommens, P.M.; Ritz, U. 3D Bioprinting for Vascularized Tissue-Engineered Bone Fabrication. Materials 2020, 13, 2278. [Google Scholar]
- Xing, H.; Lee, H.; Luo, L.; Kyriakides, T.R. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol. Adv. 2020, 42, 107421. [Google Scholar] [PubMed]
- Wu, Y.-H.A.; Chiu, Y.-C.; Lin, Y.-H.; Ho, C.-C.; Chen, Y. 3D-Printed Bioactive Calcium Silicate/Poly-ε-Caprolactone Bioscaffolds Modified with Biomimetic Extracellular Matrices for Bone Regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [PubMed] [Green Version]
- Hwangbo, H.; Lee, J.; Kim, G. Mechanically and biologically enhanced 3D-printed HA/PLLA/dECM biocomposites for bone tissue engineering. Int. J. Biol. Macromol. 2022, 218, 9–21. [Google Scholar]
- Yang, L.; Jin, S.; Shi, L.; Ullah, I.; Yu, K.; Zhang, W.; Bo, L.; Zhang, X.; Guo, X. Cryogenically 3D printed biomimetic scaffolds containing decellularized small intestinal submucosa and Sr2+/Fe3+ co-substituted hydroxyapatite for bone tissue engineering. Chem. Eng. J. 2022, 431, 133459. [Google Scholar]
- Kim, J.-Y.; Ahn, G.; Kim, C.; Lee, J.-S.; Lee, I.-G.; An, S.-H.; Yun, W.; Kim, S.-Y.; Shim, J.-H. Synergistic Effects of Beta Tri-Calcium Phosphate and Porcine-Derived Decellularized Bone Extracellular Matrix in 3D-Printed Polycaprolactone Scaffold on Bone Regeneration. Macromol. Biosci. 2018, 18, 1800025. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, J.; Kim, W.; Kim, G.H. Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering. Carbohydr. Polym. 2020, 250, 116914. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, H.; Jiang, J.; Zhao, C.; Zhang, J.; Chen, P.; Lin, X.; Fan, S. Desktop-Stereolithography 3D Printing of a Polyporous Extracellular Matrix Bioink for Bone Defect Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 589094. [Google Scholar]
- Safdari, M.; Bibak, B.; Soltani, H.; Hashemi, J. Recent advancements in decellularized matrix technology for bone tissue engineering. Differentiation 2021, 121, 25–34. [Google Scholar] [CrossRef]
- Dubey, N.; Ferreira, J.A.; Malda, J.; Bhaduri, S.B.; Bottino, M.C. Extracellular Matrix/Amorphous Magnesium Phosphate Bioink for 3D Bioprinting of Craniomaxillofacial Bone Tissue. ACS Appl. Mater. Interfaces 2020, 12, 23752–23763. [Google Scholar] [PubMed]
- Jia, L.; Hua, Y.; Zeng, J.; Liu, W.; Wang, D.; Zhou, G.; Liu, X.; Jiang, H. Bioprinting and regeneration of auricular cartilage using a bioactive bioink based on microporous photocrosslinkable acellular cartilage matrix. Bioact. Mater. 2022, 16, 66–81. [Google Scholar] [CrossRef]
- Alcala-Orozco, C.R.; Cui, X.; Hooper, G.J.; Lim, K.S.; Woodfield, T.B.F. Converging functionality: Strategies for 3D hybrid-construct biofabrication and the role of composite biomaterials for skeletal regeneration. Acta Biomater. 2021, 132, 188–216. [Google Scholar] [PubMed]
- Khati, V.; Ramachandraiah, H.; Pati, F.; Svahn, H.; Gaudenzi, G.; Russom, A. 3D Bioprinting of Multi-Material Decellularized Liver Matrix Hydrogel at Physiological Temperatures. Biosensors 2022, 12, 521. [Google Scholar] [CrossRef]
- Chae, S.; Cho, D.-W. Three-dimensional bioprinting with decellularized extracellular matrix-based bioinks in translational regenerative medicine. MRS Bull. 2022, 47, 70–79. [Google Scholar] [CrossRef]
- Lee, H.; Han, W.; Kim, H.; Ha, D.-H.; Jang, J.; Kim, B. Development of liver decellularized extracellular matrix bioink for 3D cell printing-based liver tissue engineering. Biomacromolecules 2017, 18, 1229–1237. [Google Scholar]
- Chen, Y.-W.; Shen, Y.-F.; Ho, C.-C.; Yu, J.; Wu, Y.-H.A.; Wang, K.; Shih, C.-T.; Shie, M.-Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C 2018, 91, 679–687. [Google Scholar]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, Y.; Ikeda, H.; Nagamatsu, Y.; Masaki, C.; Hosokawa, R.; Shimizu, H. PICN Nanocomposite as Dental CAD/CAM Block Comparable to Human Tooth in Terms of Hardness and Flexural Modulus. Materials 2021, 14, 1182. [Google Scholar] [CrossRef]
- Zhai, X.; Ruan, C.; Ma, Y.; Cheng, D.; Wu, M.; Liu, W.; Zhao, X.; Pan, H.; Lu, W.W. 3D-Bioprinted Osteoblast-``Laden Nanocomposite Hydrogel Constructs with Induced Microenvironments Promote Cell Viability, Differentiation, and Osteogenesis both In Vitro and In Vivo. Adv. Sci. 2018, 5, 1700550. [Google Scholar] [CrossRef] [Green Version]
- Park, H.; Lee, J.-H.; Lee, S. The comprehensive on-demand 3D bio-printing for composite reconstruction of mandibular defects. Maxillofac. Plast. Reconstr. Surg. 2022, 44, 31. [Google Scholar] [PubMed]
- Park, J.; Lee, S.J.; Jo, H.H.; Lee, J.H.; Kim, W.D.; Lee, J.Y.; Park, S.A. Fabrication and characterization of 3D-printed bone-like β-tricalcium phosphate/polycaprolactone scaffolds for dental tissue engineering. J. Ind. Eng. Chem. 2017, 46, 175–181. [Google Scholar]
- Bhattacharjee, A.; Bose, S. 3D printed hydroxyapatite–Zn2+ functionalized starch composite bone grafts for orthopedic and dental applications. Mater. Des. 2022, 221, 110903. [Google Scholar] [CrossRef]
- Sun, J.; Yu, J.; Wade-Zhu, J.; Wang, Y.; Qu, H.; Zhao, S.; Zhang, R.; Yang, J.; Binner, J.; Bai, J. 3d Printing of Ceramic Composite with Biomimetic Toughening Design. SSRN Electron. J. 2022, 58, 103027. [Google Scholar]
- Han, J.; Kim, D.S.; Jang, H.; Kim, H.-R.; Kang, H.-W. Bioprinting of three-dimensional dentin–pulp complex with local differentiation of human dental pulp stem cells. J. Tissue Eng. 2019, 10, 2041731419845849. [Google Scholar] [PubMed]
- Machla, F.; Angelopoulos, I.; Epple, M.; Chatzinikolaidou, M.; Bakopoulou, A. Biomolecule-Mediated Therapeutics of the Dentin-Pulp Complex: A Systematic Review. Biomolecules 2022, 12, 285. [Google Scholar] [CrossRef]
- Sharma, V.; Dash, S.K.; Govarthanan, K.; Gahtori, R.; Negi, N.; Barani, M.; Tomar, R.; Chakraborty, S.; Mathapati, S.; Bishi, D.K.; et al. Recent Advances in Cardiac Tissue Engineering for the Management of Myocardium Infarction. Cells 2021, 10, 2538. [Google Scholar] [PubMed]
- Zhang, Y.S.; Arneri, A.; Bersini, S.; Shin, S.; Zhu, K.; Goli-Malekabadi, Z.; Aleman, J.; Colosi, C.; Busignani, F.; Dell Erba, V.; et al. Bioprinting 3D Microfibrous Scaffolds for Engineering Endothelialized Myocardium and Heart-on-a-Chip. Biomaterials 2016, 110, 45–59. [Google Scholar] [PubMed] [Green Version]
- Zhang, Y.S.; Pi, Q.; van Genderen, A.M. Microfluidic Bioprinting for Engineering Vascularized Tissues and Organoids. J. Vis. Exp. 2017, 2017, e55957. [Google Scholar]
- Wang, L.; Cao, Y.; Shen, Z.; Li, M.; Zhang, W.; Liu, Y.; Zhang, Y.; Duan, J.; Ma, Z.; Sang, S. 3D printed GelMA/carboxymethyl chitosan composite scaffolds for vasculogenesis. Int. J. Polym. Mater. Polym. Biomater. 2022, 1–13. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Y.; Li, S.; Yuanshao, Y.; Li, y.; Jiang, C.; Qingxi, h. Bioprinted Chitosan and Hydroxyapatite Micro-Channels Structures Scaffold for Vascularization of Bone Regeneration. J. Biomater. Tissue Eng. 2017, 7, 28–34. [Google Scholar]
- Yeo, M.; Kim, G. Micro/nano-hierarchical scaffold fabricated using a cell electrospinning/3D printing process for co-culturing myoblasts and HUVECs to induce myoblast alignment and differentiation. Acta Biomater. 2020, 107, 102–114. [Google Scholar] [CrossRef]
- Zou, Q.; Grottkau, B.; He, Z.; Shu, L.; Yang, L.; Ma, M.; Ye, C. Biofabrication of valentine-shaped heart with a composite hydrogel and sacrificial material. Mater. Sci. Eng. C 2019, 108, 110205. [Google Scholar] [CrossRef]
- Frost, B.; Sutliff, B.; Thayer, P.; Bortner, M.; Foster, E.J. Gradient Poly(ethylene glycol) Diacrylate and Cellulose Nanocrystals Tissue Engineering Composite Scaffolds via Extrusion Bioprinting. Front. Bioeng. Biotechnol. 2019, 7, 280. [Google Scholar] [PubMed] [Green Version]
- Suntornnond, R.; Tan, E.Y.S.; An, J.; Chua, C.K. A highly printable and biocompatible hydrogel composite for direct printing of soft and perfusable vasculature-like structures. Sci. Rep. 2017, 7, 16902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hockaday, L.; Armstrong, P.; Lee, L.; Duan, B.; Kang, K.; Butcher, J. Optimizing Photo-Encapsulation Viability of Heart Valve Cell Types in 3D Printable Composite Hydrogels. Ann. Biomed. Eng. 2016, 45, 360–377. [Google Scholar]
- Wu, Y.; Heikal, L.; Ferns, G.; Ghezzi, P.; Nokhodchi, A.; Maniruzzaman, M. 3D Bioprinting of Novel Biocompatible Scaffolds for Endothelial Cell Repair. Polymers 2019, 11, 1924. [Google Scholar] [PubMed] [Green Version]
- Cunniffe, G.; Gonzalez-Fernandez, T.; Daly, A.; Nelson Sathy, B.; Jeon, O.; Alsberg, E.; Kelly, D. Three-Dimensional Bioprinting of Polycaprolactone Reinforced Gene Activated Bioinks for Bone Tissue Engineering. Tissue Eng. Part A 2017, 23, 891–900. [Google Scholar]
- Park, J.Y.; Jang, J.; Kang, H.-W. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Yu, F.; Choudhury, D. Microfluidic bioprinting for organ-on-a-chip models. Drug Discov. Today 2019, 24, 1248–1257. [Google Scholar]
- Arrigoni, C.; Gilardi, M.; Bersini, S.; Candrian, C.; Moretti, M. Bioprinting and Organ-on-Chip Applications Towards Personalized Medicine for Bone Diseases. Stem Cell Rev. Rep. 2017, 13, 407–417. [Google Scholar] [PubMed]
- Lee, H. One-step fabrication of an organ-on-a-chip with spatial heterogeneity using a 3D bioprinting technology. Lab A Chip 2016, 16, 2618–2625. [Google Scholar] [CrossRef] [Green Version]
- Shery Huang, Y.Y.; Zhang, D.; Liu, Y. Bioprinting of three-dimensional culture models and organ-on-a-chip systems. MRS Bull. 2017, 42, 593–599. [Google Scholar]
- Aazmi, A.; Zhou, H.; Li, Y.; Yu, M.; Xu, X.; Wu, Y.; Ma, L.; Zhang, B.; Yang, H. Engineered Vasculature for Organ-on-a-Chip Systems. Engineering 2022, 9, 131–147. [Google Scholar] [CrossRef]
- Abudupataer, M.; Chen, N.; Yan, S.; Alam, F.; Shi, Y.; Wang, L.; Lai, H.; Li, J.; Zhu, K.; Wang, C. Bioprinting a 3D vascular construct for engineering a vessel-on-a-chip. Biomed. Microdevices 2019, 22, 10. [Google Scholar] [CrossRef]
- Kissinger, P.T. Biosensors—A perspective. Biosens. Bioelectron. 2005, 20, 2512–2516. [Google Scholar] [CrossRef]
- Phumlani, T.; Poslet Morgan, S.; Zikhona, N.-T. Biosensors: Design, Development and Applications. In Nanopores; Sadia, A., Akhtar, M.S., Hyung-Shik, S., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Dias, A.; Kingsley, D.; Corr, D. Recent Advances in Bioprinting and Applications for Biosensing. Biosensors 2014, 4, 111–136. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Li, Z.; Wang, X.; Tian, F.; Yang, J. Surface-Fabrication of Fluorescent Hydroxyapatite for Cancer Cell Imaging and Bio-Printing Applications. Biosensors 2022, 12, 419. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gao, G.; Qiu, Y. Accelerated myotube formation using bioprinting technology for biosensor applications. Biotechnol. Lett. 2012, 35, 315–321. [Google Scholar] [CrossRef] [PubMed]



| 3D Bioprinting Method | Physical Principle | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Extrusion-based 3D bioprinting | The most conventional 3D bioprinting technique, based on the use of varying pressure and temperature values to formulate bioprinted constructs of hierarchical architecture. | Continuous extrusion reinforces the robustness of the scaffolds; Tackles viscosity issues more efficaciously. | High stresses and temperatures developed during fabrication can adversely affect cell viability. | [21,127,129] |
| Inkjet 3D bioprinting | A method that does not require direct contact, utilizing piezoelectric, thermal, and electromagnetic sources in order to direct the ejection of multiple bioink droplets into different 3D shapes. | High printing fidelity; High printing velocity; Facilitates the miscibility of different biomolecules; Insubstantial effect on cells viability during printing. | Only low-viscosity bioinks are printable. | [28,132,133] |
| Stereolithography 3D bioprinting | A technique that relies on the crosslinking of a photopolymerizable bioink solution, after its pouring into a mold with desired geometrical properties and its solidification under the irradiation from either a laser or UV light source. | High spatial resolution; Use of predesigned molds enhances printing fidelity. | Slow process because it consists of two phases, UV and laser irradiation can damage cells; The dispersed nanophase can affect the extent of photopolymerization due to light scattering. | [6,27] |
| Laser-assisted 3D bioprinting | A laser beam is guided toward sequential bioink droplets, resulting in heating them and, eventually, leading to their deposition on a surface, without requiring direct contact with this target area. | Fast printing speed. Allows for high initial cell number entrapment in the bioink droplet. | Laser beam can potentially harm cells due to the heat absorbed by the droplet. | [27,65,138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukelis, K.; Helal, Z.A.; Mikos, A.G.; Chatzinikolaidou, M. Nanocomposite Bioprinting for Tissue Engineering Applications. Gels 2023, 9, 103. https://doi.org/10.3390/gels9020103
Loukelis K, Helal ZA, Mikos AG, Chatzinikolaidou M. Nanocomposite Bioprinting for Tissue Engineering Applications. Gels. 2023; 9(2):103. https://doi.org/10.3390/gels9020103
Chicago/Turabian StyleLoukelis, Konstantinos, Zina A. Helal, Antonios G. Mikos, and Maria Chatzinikolaidou. 2023. "Nanocomposite Bioprinting for Tissue Engineering Applications" Gels 9, no. 2: 103. https://doi.org/10.3390/gels9020103
APA StyleLoukelis, K., Helal, Z. A., Mikos, A. G., & Chatzinikolaidou, M. (2023). Nanocomposite Bioprinting for Tissue Engineering Applications. Gels, 9(2), 103. https://doi.org/10.3390/gels9020103