Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications
Abstract
:1. Introduction
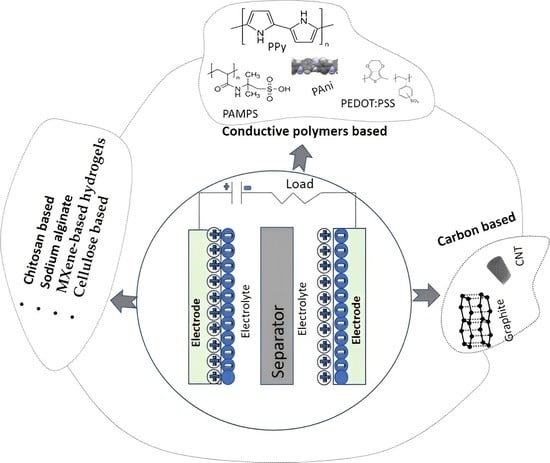
2. Hydrogels
3. Hydrogels for Supercapacitor Production
3.1. Electrochemical Properties of Hydrogels
3.2. Carbon-Based Hydrogels
3.2.1. Graphene-Based Hydrogels
3.2.2. Carbon Nanotubes
3.3. Conductive Polymer-Based Hydrogels
3.3.1. PAMPS-Based Hydrogels
3.3.2. Polyaniline (PAni) Hydrogel
3.3.3. Poly(3,4-ethylenedioxythiophene) Polystyrene Sulfonate-Based Hydrogel
3.3.4. Polypyrrole (PPy)-Based Hydrogels
3.4. Cellulose-Based Hydrogels for Supercapacitor Applications
3.5. Other Hydrogels for Supercapacitor Production
3.5.1. Chitosan-Based Hydrogels
3.5.2. Sodium Alginate
3.5.3. MXene-Based Hydrogels
4. Challenges of Hydrogels for Flexible Supercapacitors
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peppas, N.A. Hydrogels and drug delivery. Curr. Opin. Colloid Interface Sci. 1997, 2, 531–537. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Agarwal, R.; Alam, M. Hydrogels for Wound Healing Applications. In Biomedical Hydrogels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 184–227. [Google Scholar]
- Zohuriaan-Mehr, M.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. JMatS 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Tien, H.N.; Hien, N.T.M.; Oh, E.-S.; Chung, J.; Kim, E.J.; Choi, W.M.; Kong, B.-S.; Hur, S.H. Synthesis of a highly conductive and large surface area graphene oxide hydrogel and its use in a supercapacitor. J. Mater. Chem. A 2013, 1, 208–211. [Google Scholar]
- Tadesse, M.G.; Kasaw, E.; Fentahun, B.; Loghin, E.; Lübben, J.F. Banana Peel and Conductive Polymers-Based Flexible Supercapacitors for Energy Harvesting and Storage. Energies 2022, 15, 2471. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Loghin, C.; Dulgheriu, I.; Loghin, E. Comfort Evaluation of Wearable Functional Textiles. Materials 2021, 14, 6466. [Google Scholar] [CrossRef]
- Blažic, R.; Kučić Grgić, D.; Kraljić Roković, M.; Vidović, E. Cellulose-g-poly (2-(dimethylamino) ethylmethacrylate) Hydrogels: Synthesis, Characterization, Antibacterial Testing and Polymer Electrolyte Application. Gels 2022, 8, 636. [Google Scholar] [CrossRef]
- Zhang, X. Heat-storage and thermo-regulated textiles and clothing. Smart Fibres Fabr. Cloth. 2001, 2001, 34–57. [Google Scholar]
- Park, S.; Jayaraman, S. Smart textiles: Wearable electronic systems. MRS Bull. 2003, 28, 585–591. [Google Scholar] [CrossRef]
- Hossain, I.Z.; Khan, A.; Hossain, G. A Piezoelectric Smart Textile for Energy Harvesting and Wearable Self-Powered Sensors. Energies 2022, 15, 5541. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Mengistie, D.A.; Chen, Y.; Wang, L.; Loghin, C.; Nierstrasz, V. Electrically conductive highly elastic polyamide/lycra fabric treated with PEDOT: PSS and polyurethane. JMatS 2019, 54, 9591–9602. [Google Scholar]
- Tadesse, M.G.; Dumitrescu, D.; Loghin, C.; Chen, Y.; Wang, L.; Nierstrasz, V. 3D printing of NinjaFlex filament onto PEDOT: PSS-coated textile fabrics for electroluminescence applications. J. Electron. Mater. 2018, 47, 2082–2092. [Google Scholar] [CrossRef] [Green Version]
- Jost, K.; Perez, C.R.; McDonough, J.K.; Presser, V.; Heon, M.; Dion, G.; Gogotsi, Y. Carbon coated textiles for flexible energy storage. Energy Environ. Sci. 2011, 4, 5060–5067. [Google Scholar] [CrossRef]
- Fang, Y.; Chen, G.; Bick, M.; Chen, J. Smart textiles for personalized thermoregulation. Chem. Soc. Rev. 2021, 50, 9357–9374. [Google Scholar] [CrossRef]
- Niu, Z.; Qi, S.; Shuaib, S.S.A.; Yuan, W. Flexible, stimuli-responsive and self-cleaning phase change fiber for thermal energy storage and smart textiles. Compos. Part B 2022, 228, 109431. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Choi, K.-H.; Choi, W.-S.; Kwon, Y.H.; Jung, H.-R.; Shin, H.-C.; Kim, J.Y. Progress in flexible energy storage and conversion systems, with a focus on cable-type lithium-ion batteries. Energy Environ. Sci. 2013, 6, 2414–2423. [Google Scholar] [CrossRef]
- Tebyetekerwa, M.; Marriam, I.; Xu, Z.; Yang, S.; Zhang, H.; Zabihi, F.; Jose, R.; Peng, S.; Zhu, M.; Ramakrishna, S. Critical insight: Challenges and requirements of fibre electrodes for wearable electrochemical energy storage. Energy Environ. Sci. 2019, 12, 2148–2160. [Google Scholar] [CrossRef]
- Lu, Z.; Raad, R.; Safaei, F.; Xi, J.; Liu, Z.; Foroughi, J. Carbon nanotube based fiber supercapacitor as wearable energy storage. Front. Mater. 2019, 6, 138. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage–A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Wen, Z.; Yeh, M.-H.; Guo, H.; Wang, J.; Zi, Y.; Xu, W.; Deng, J.; Zhu, L.; Wang, X.; Hu, C. Self-powered textile for wearable electronics by hybridizing fiber-shaped nanogenerators, solar cells, and supercapacitors. Sci. Adva. 2016, 2, e1600097. [Google Scholar] [CrossRef] [Green Version]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Zhao, C.; Wan, T.; Yuan, W.; Zheng, Z.; Jia, X.; Shu, K.; Feng, L.; Min, Y. Re-Stickable Yarn Supercapacitors with Vaper Phase Polymerized Multi-Layered Polypyrrole Electrodes for Smart Garments. Macromol. Rapid Commun. 2022, 43, 2200347. [Google Scholar] [CrossRef] [PubMed]
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A wearable supercapacitor based on conductive PEDOT: PSS-coated cloth and a sweat electrolyte. Adv. Mater. 2020, 32, 1907254. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lu, X. Functional hydrogel-based supercapacitors for wearable bioelectronic devices. Mater. Chem. Front. 2021, 5, 7479–7498. [Google Scholar] [CrossRef]
- Guo, Y.; Bae, J.; Zhao, F.; Yu, G. Functional hydrogels for next-generation batteries and supercapacitors. Trends Chem. 2019, 1, 335–348. [Google Scholar] [CrossRef]
- Rong, Q.; Lei, W.; Liu, M. Conductive hydrogels as smart materials for flexible electronic devices. Chem. A Eur. J. 2018, 24, 16930–16943. [Google Scholar] [CrossRef]
- Sultana, S.; Ahmed, K.; Jiwanti, P.K.; Wardhana, B.Y.; Shiblee, M.N.I. Ionic liquid-based gels for applications in electrochemical energy storage and conversion devices: A review of recent progress and future prospects. Gels 2021, 8, 2. [Google Scholar] [CrossRef]
- Hasan, K.; Bashir, S.; Subramaniam, R.; Kasi, R.; Kamran, K.; Iqbal, J.; Algharni, H.; Al-Sehemi, A.G.; Wageh, S.; Pershaanaa, M. Poly (Vinyl Alcohol)/Agar Hydrogel Electrolytes Based Flexible All-in-One Supercapacitors with Conducting Polyaniline/Polypyrrole Electrodes. Polymers 2022, 14, 4784. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen-and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef]
- Heidarian, P.; Kouzani, A.Z.; Kaynak, A.; Paulino, M.; Nasri-Nasrabadi, B.; Zolfagharian, A.; Varley, R. Dynamic plant-derived polysaccharide-based hydrogels. Carbohydr. Polym. 2020, 231, 115743. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-forming algae polysaccharides: From seaweed to biomedical applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Kabir, S.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.; Ali, A.; Islam, M. Cellulose-based hydrogel materials: Chemistry, properties and their prospective applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, P.; Chen, J.; Sun, Z.; Zhao, B. Electrically conductive hydrogels for flexible energy storage systems. Prog. Polym. Sci. 2019, 88, 220–240. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, C.; Sun, N.; Tan, D.; Li, Q.; Bi, S.; Song, J. Recent progress in multifunctional hydrogel-based supercapacitors. J. Sci. Adv. Mater. Devices 2021, 6, 338–350. [Google Scholar] [CrossRef]
- Majee, S.B. Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: Rijeka, Croatia, 2016; pp. 232–248. [Google Scholar]
- Meng, X.; Sun, H.; Zhu, J.; Bi, H.; Han, Q.; Liu, X.; Wang, X. Graphene-based cobalt sulfide composite hydrogel with enhanced electrochemical properties for supercapacitors. New J. Chem. 2016, 40, 2843–2849. [Google Scholar] [CrossRef]
- Khan, Y.; Bashir, S.; Hina, M.; Ramesh, S.; Ramesh, K.; Mujtaba, M.; Lahiri, I. Effect of charge density on the mechanical and electrochemical properties of poly (acrylic acid) hydrogel electrolytes based flexible supercapacitors. Mater. Today Commun. 2020, 25, 101558. [Google Scholar] [CrossRef]
- Tadesse, M.G.; Loghin, C.; Chen, Y.; Wang, L.; Catalin, D.; Nierstrasz, V. Effect of liquid immersion of PEDOT: PSS-coated polyester fabric on surface resistance and wettability. Smart Mater. Struct. 2017, 26, 065016. [Google Scholar] [CrossRef]
- Anjali, J.; Jose, V.K.; Lee, J.-M. Carbon-based hydrogels: Synthesis and their recent energy applications. J. Mater. Chem. A 2019, 7, 15491–15518. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-performance supercapacitors based on the carbon nanotubes, graphene and graphite nanoparticles electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaucamp, A.; Muddasar, M.; Crawford, T.; Collins, M.N.; Culebras, M. Sustainable lignin precursors for tailored porous carbon-based supercapacitor electrodes. Int. J. Biol. Macromol. 2022, 221, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Xu, W.; Fang, L.; Duan, J. High performance of functionalized graphene hydrogels using ethylenediamine for supercapacitor applications. Front. Chem. 2022, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. Graphite nanoparticles paper supercapacitor based on gel electrolyte. Mater. Renew. Sustain. Energy 2018, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.G.; Hong, J.; Hong, W.H.; Hammond, P.T.; Park, H. Facilitated ion transport in all-solid-state flexible supercapacitors. ACS Nano 2011, 5, 7205–7213. [Google Scholar] [CrossRef]
- Banda, H.; Aradilla, D.; Benayad, A.; Chenavier, Y.; Daffos, B.; Dubois, L.; Duclairoir, F. One-step synthesis of highly reduced graphene hydrogels for high power supercapacitor applications. J. Power Source 2017, 360, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Chabi, S.; Xia, Y.; Zhu, Y. Preparation of 3D graphene-based architectures and their applications in supercapacitors. Prog. Nat. Sci. Mater. Int. 2015, 25, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Duan, J.; Tang, Y.; Zhang Qiao, S. Hybrid hydrogels of porous graphene and nickel hydroxide as advanced supercapacitor materials. Chem. A Eur. J. 2013, 19, 7118–7124. [Google Scholar] [CrossRef]
- Jiang, C.; Gao, M.; Zhang, S.; Huang, L.; Yu, S.; Song, Z.; Wu, Q. Chitosan/graphene oxide hybrid hydrogel electrode with porous network boosting ultrahigh energy density flexible supercapacitor. Int. J. Biol. Macromol. 2022, 225, 1437–1448. [Google Scholar] [CrossRef]
- Ren, R.; Zhong, Y.; Ren, X.; Fan, Y. Chitosan-based oxygen-doped activated carbon/graphene composite for flexible supercapacitors. RSC Adv. 2022, 12, 25807–25814. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, G.Y.; Lim, J.; Kim, S.O. CNT–rGO Hydrogel-Integrated Fabric Composite Synthesized via an Interfacial Gelation Process for Wearable Supercapacitor Electrodes. ACS Omega 2021, 6, 19578–19585. [Google Scholar] [CrossRef]
- Liu, J.; Khanam, Z.; Ahmed, S.; Wang, T.; Wang, H.; Song, S. Flexible antifreeze zn-ion hybrid supercapacitor based on gel electrolyte with graphene electrodes. ACS Appl. Mater. Interfaces 2021, 13, 16454–16468. [Google Scholar] [CrossRef]
- Barzegar, F.; Pavlenko, V.; Zahid, M.; Bello, A.; Xia, X.; Manyala, N.; Ozoemena, K.I.; Abbas, Q. Tuning the nanoporous structure of carbons derived from the composite of cross-linked polymers for charge storage applications. ACS Appl. Energy Mater. 2021, 4, 1763–1773. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Zhang, K.; Shao, J.; Qiu, J.; Wu, J.; Wu, Y.; Yan, L. A high-voltage quasi-solid-state flexible supercapacitor with a wide operational temperature range based on a low-cost “water-in-salt” hydrogel electrolyte. Nanoscale 2021, 13, 3010–3018. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, S.; Xiao, H.; Zhou, X.; Xu, X.; Yang, J.; Siddique, A.H.; Liu, Z. Graphene modified polyaniline-hydrogel based stretchable supercapacitor with high capacitance and excellent stretching stability. ChemSusChem 2021, 14, 938–945. [Google Scholar] [CrossRef]
- Wang, H.; Deng, Y.; Qiu, J.; Wu, J.; Zhang, K.; Shao, J.; Yan, L. In Situ Formation of “Dimethyl Sulfoxide/Water-in-Salt”-Based Chitosan Hydrogel Electrolyte for Advanced All-Solid-State Supercapacitors. ChemSusChem 2021, 14, 632–641. [Google Scholar] [CrossRef]
- You, Y.; Qu, K.; Shi, C.; Sun, Z.; Huang, Z.; Li, J.; Dong, M.; Guo, Z. Binder-free CuS/ZnS/sodium alginate/rGO nanocomposite hydrogel electrodes for enhanced performance supercapacitors. Int. J. Biol. Macromol. 2020, 162, 310–319. [Google Scholar] [CrossRef]
- Deng, Y.; Shang, T.; Wu, Z.; Tao, Y.; Luo, C.; Liang, J.; Han, D.; Lyu, R.; Qi, C.; Lv, W. Fast gelation of Ti3C2Tx MXene initiated by metal ions. Adv. Mater. 2019, 31, 1902432. [Google Scholar] [CrossRef]
- Han, M.; Jayakumar, A.; Li, Z.; Zhao, Q.; Zhang, J.; Jiang, X.; Guo, X.; Wang, R.; Xu, C.; Song, S. Fabricating 3D macroscopic graphene-based architectures with outstanding flexibility by the novel liquid drop/colloid flocculation approach for energy storage applications. ACS Appl. Mater. Interfaces 2018, 10, 21991–22001. [Google Scholar] [CrossRef]
- Zheng, L.; Guan, L.; Yang, G.; Chen, S.; Zheng, H. One-pot synthesis of CoFe 2 O 4/rGO hybrid hydrogels with 3D networks for high capacity electrochemical energy storage devices. RSC Adv. 2018, 8, 8607–8614. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, L.; Wang, X.; Ok, Y.S.; Elliott, J.A.; Chang, S.X.; Chung, H.-J. Flexible and self-healing aqueous supercapacitors for low temperature applications: Polyampholyte gel electrolytes with biochar electrodes. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Gao, A.; Song, X.; Shu, D.; Yi, F.; Zhong, J.; Zeng, R.; Zhao, S.; Meng, T. Supramolecule-inspired fabrication of carbon nanoparticles in situ anchored graphene nanosheets material for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 26775–26782. [Google Scholar] [CrossRef] [PubMed]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pour, G.B.; Aval, L.F.; Mirzaee, M. CNTs Supercapacitor Based on the PVDF/PVA Gel Electrolytes. Recent Pat. Nanotechnol. 2020, 14, 163–170. [Google Scholar] [CrossRef]
- Hu, Y.; Zhan, Y.; Xu, M.; Niu, F.; Chen, Y.; Yang, Q.; Xiong, C.; Shi, Z. One-step preparation of flexible nanocellulose-based composite hydrogel supercapacitors with high specific capacitance. Compos. Sci. Technol. 2022, 230, 109725. [Google Scholar] [CrossRef]
- Gilshtein, E.; Flox, C.; Ali, F.S.; Mehrabimatin, B.; Fedorov, F.S.; Lin, S.; Zhao, X.; Nasibulin, A.G.; Kallio, T. Superior environmentally friendly stretchable supercapacitor based on nitrogen-doped graphene/hydrogel and single-walled carbon nanotubes. J. Energy Storage 2020, 30, 101505. [Google Scholar] [CrossRef]
- Lyu, L.; Kang, J.; Seong, K.-d.; Kim, C.W.; Lim, J.; Piao, Y. ZnNiCo hydroxide/graphene-carbon nanotube hydrogel on surface-modified Ni foam as a battery-type electrode for hybrid supercapacitors. J. Alloy. Compd. 2021, 872, 159610. [Google Scholar] [CrossRef]
- Han, J.; Wang, H.; Yue, Y.; Mei, C.; Chen, J.; Huang, C.; Wu, Q.; Xu, X. A self-healable and highly flexible supercapacitor integrated by dynamically cross-linked electro-conductive hydrogels based on nanocellulose-templated carbon nanotubes embedded in a viscoelastic polymer network. Carbon 2019, 149, 1–18. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, S.; Geng, A.; Wang, L.; Song, C.; Xu, L.; Jia, C.; Shi, J.; Gan, L. Using TEMPO-oxidized-nanocellulose stabilized carbon nanotubes to make pigskin hydrogel conductive as flexible sensor and supercapacitor electrode: Inspired from a Chinese cuisine. Compos. Sci. Technol. 2020, 196, 108226. [Google Scholar] [CrossRef]
- Dadashi, R.; Bahram, M.; Faraji, M. Fabrication of a solid-state symmetrical supercapacitor based on polyaniline grafted multiwalled carbon nanotube deposit onto Created Vertically Oriented Graphene Nanosheets on graphite sheet. J. Energy Storage 2022, 52, 104775. [Google Scholar] [CrossRef]
- Xu, H.; Cui, L.; Pan, X.; An, Y.; Jin, X. Carboxymethylcellulose-polyaniline/carbon nanotube (CMC-PANI/CNT) film as flexible and highly electrochemical active electrode for supercapacitors. Int. J. Biol. Macromol. 2022, 219, 1135–1145. [Google Scholar] [CrossRef]
- Thanasamy, D.; Jesuraj, D.; Avadhanam, V.; Chinnadurai, K.; Kannan, S.K.K. Microstructural effect of various polyaniline-carbon nanotube core-shell nanocomposites on electrochemical supercapacitor electrode performance. J. Energy Storage 2022, 53, 105087. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Wang, F.; Zhang, J.H.; Zhang, J.; Shi, Y.; Pan, L. Application of conductive polymer hydrogels in flexible electronics. J. Polym. Sci. 2022, 60, 2635–2662. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Pi, M.; Ran, R. High Performance Double Conductive Network Hydrogel Based on Soaking Strategy for Supercapacitors. Macromol. Mater. Eng. 2022, 307, 2100652. [Google Scholar] [CrossRef]
- Cevik, E.; Bozkurt, A.; Hassan, M.; Gondal, M.A.; Qahtan, T.F. Redox-mediated poly (2-acrylamido-2-methyl-1-propanesulfonic acid)/ammonium molybdate hydrogels for highly effective flexible supercapacitors. ChemElectroChem 2019, 6, 2876–2882. [Google Scholar] [CrossRef]
- Afrifah, V.A.; Phiri, I.; Hamenu, L.; Madzvamuse, A.; Lee, K.S.; Ko, J.M. Electrochemical properties of poly (2-acrylamido-2-methylpropane sulfonic acid) polyelectrolyte containing zwitterionic silica sulfobetaine for supercapacitors. J. Power Source 2020, 479, 228657. [Google Scholar] [CrossRef]
- Bozkurt, A.; Cevik, E. Supercapacitor Based on Polymer Electrolyte Containing MO (iv) Doped Hydrogel. U.S. Patent 16/660,267, 22 April 2021. [Google Scholar]
- Yazar, S.; Atun, G. Electrochemical synthesis of tunable polypyrrole-based composites on carbon fabric for wide potential window aqueous supercapacitor. IJER 2022, 46, 14408–14423. [Google Scholar] [CrossRef]
- Kim, K.M.; Nam, J.H.; Lee, Y.-G.; Cho, W.I.; Ko, J.M. Supercapacitive properties of electrodeposited RuO2 electrode in acrylic gel polymer electrolytes. CAP 2013, 13, 1702–1706. [Google Scholar] [CrossRef]
- Cevik, E.; Gunday, S.T.; Akhtar, S.; Bozkurt, A. A comparative study of various polyelectrolyte/nanocomposite electrode combinations in symmetric supercapacitors. Int. J. Hydrog. Energy 2019, 44, 16099–16109. [Google Scholar] [CrossRef]
- Jung, G.; Lee, H.; Park, H.; Kim, J.; Kim, J.W.; Kim, D.S.; Keum, K.; Lee, Y.H.; Ha, J.S. Temperature-tolerant flexible supercapacitor integrated with a strain sensor using an organohydrogel for wearable electronics. Chem. Eng. J. 2022, 450, 138379. [Google Scholar] [CrossRef]
- Kalam, A.A.; Yoo, J.E.; Bae, J. Novel application of water-dispersible polyaniline-poly (2-acrylamido-2-methyl-1-propanesulfonic acid) for all-solution-based electrochemical capacitors. Polym. Test. 2015, 44, 49–56. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Alesary, H.F.; Ismail, H.K.; Khudhair, A.F.; Mohammed, M.Q. Effects of dopant ions on the properties of polyaniline conducting polymer. Orient. J. Chem. 2018, 34, 2525. [Google Scholar] [CrossRef] [Green Version]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.C.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Cao, L.; Lai, F.; Wang, X.; Huang, S.; Du, X.; Li, W.; Lin, Z.; Zhang, P. Double-cross-linked polyaniline hydrogel and its application in supercapacitors. Ionics 2022, 28, 423–432. [Google Scholar] [CrossRef]
- Joo, H.; Han, H.; Cho, S. Fabrication of poly (vinyl alcohol)-polyaniline nanofiber/graphene hydrogel for high-performance coin cell supercapacitor. Polymers 2020, 12, 928. [Google Scholar] [CrossRef] [Green Version]
- Dou, P.; Liu, Z.; Cao, Z.; Zheng, J.; Wang, C.; Xu, X. Rapid synthesis of hierarchical nanostructured Polyaniline hydrogel for high power density energy storage application and three-dimensional multilayers printing. JMatS 2016, 51, 4274–4282. [Google Scholar] [CrossRef]
- Suganya, N.; Jaisankar, V.; Sivakumar, E. Conducting polymeric hydrogel electrolyte based on carboxymethylcellulose and polyacrylamide/polyaniline for supercapacitor applications. IJN 2018, 17, 1760003. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Maryum, P.; Wang, Q.; Zhu, J.; Min, F.; Cheng, H.; Zhao, S.; Wang, C. Bifunctional polyaniline electroconductive hydrogels with applications in supercapacitor and wearable strain sensors. J. Biomater. Sci. Polym. Ed. 2020, 31, 938–953. [Google Scholar] [CrossRef]
- Alhashmi Alamer, F.; Althagafy, K.; Alsalmi, O.; Aldeih, A.; Alotaiby, H.; Althebaiti, M.; Alghamdi, H.; Alotibi, N.; Saeedi, A.; Zabarmawi, Y. Review on PEDOT: PSS-Based Conductive Fabric. ACS Omega 2022, 7, 35371–35386. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, J.; Yang, C.; Zang, L.; Zhang, G.; Sakai, E. High-performance PVA/PEDOT: PSS hydrogel electrode for all-gel-state flexible supercapacitors. Adv. Mater. Technol. 2021, 6, 2000919. [Google Scholar] [CrossRef]
- Li, J.; Yan, W.; Zhang, G.; Sun, R.; Ho, D. Natively stretchable micro-supercapacitors based on a PEDOT: PSS hydrogel. J. Mater. Chem. C 2021, 9, 1685–1692. [Google Scholar] [CrossRef]
- Chao, Y.; Ge, Y.; Chen, Z.; Cui, X.; Zhao, C.; Wang, C.; Wallace, G.G. One-pot hydrothermal synthesis of solution-processable MoS2/PEDOT: PSS composites for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 7285–7296. [Google Scholar] [CrossRef]
- Xu, D.; Feng, X.; Niu, D.; Zhu, X.; Song, Y. PEDOT: PSS hydrogel film for supercapacitors via AlCl3-induced cross-linking and subsequent organic solvent treatments. Mater. Today Commun. 2020, 24, 101090. [Google Scholar] [CrossRef]
- Hina, M.; Bashir, S.; Kamran, K.; Iqbal, J.; Ramesh, S.; Ramesh, K. Fabrication of aqueous solid-state symmetric supercapacitors based on self-healable poly (acrylamide)/PEDOT: PSS composite hydrogel electrolytes. Mater. Chem. Phys. 2021, 273, 125125. [Google Scholar] [CrossRef]
- Shih, C.-C.; Lin, Y.-C.; Gao, M.; Wu, M.; Hsieh, H.-C.; Wu, N.-L.; Chen, W.-C. A rapid and green method for the fabrication of conductive hydrogels and their applications in stretchable supercapacitors. J. Power Source 2019, 426, 205–215. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-conductivity polymer hydrogels with arbitrary structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, J.; Bai, B.; Qiu, A.; Losic, D.; Shi, D.; Chen, M. Free-standing PEDOT/polyaniline conductive polymer hydrogel for flexible solid-state supercapacitors. Electrochim. Acta 2019, 322, 134769. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, D.; Dong, W.; Chen, M. Self-Standing Hydrogels Composed of Conducting Polymers for All-Hydrogel-State Supercapacitors. Chem. A Eur. J. 2020, 26, 1846–1855. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, Q.; Chen, W.; Yi, X.; Liu, S.; Wang, Q.; Liu, Y.; Li, J.; Li, X.; Yu, H. Highly flexible and conductive cellulose-mediated PEDOT: PSS/MWCNT composite films for supercapacitor electrodes. ACS Appl. Mater. Interfaces 2017, 9, 13213–13222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Teng, W.; Jin, Y.; Sun, L.; Hu, P.; Li, H.; Wang, L.; Wang, J. Highly-conductive PEDOT: PSS hydrogel framework based hybrid fiber with high volumetric capacitance and excellent rate capability. Electrochim. Acta 2020, 334, 135530. [Google Scholar] [CrossRef]
- Sardana, S.; Gupta, A.; Singh, K.; Maan, A.; Ohlan, A. Conducting polymer hydrogel based electrode materials for supercapacitor applications. J. Energy Storage 2021, 45, 103510. [Google Scholar] [CrossRef]
- Cheng, T.; Zhang, Y.Z.; Wang, S.; Chen, Y.L.; Gao, S.Y.; Wang, F.; Lai, W.Y.; Huang, W. Conductive hydrogel-based electrodes and electrolytes for stretchable and self-healable supercapacitors. Adv. Funct. Mater. 2021, 31, 2101303. [Google Scholar] [CrossRef]
- Mirzaee, M.; Pour, G.B. Design and fabrication of ultracapacitor based on paper substrate and BaTiO3/PEDOT: PSS separator film. Recent Pat. Nanotechnol. 2018, 12, 192–199. [Google Scholar] [CrossRef]
- Murray, P.; Spinks, G.; Wallace, G.; Burford, R. In-situ mechanical properties of tosylate doped (pTS) polypyrrole. Synth. Met. 1997, 84, 847–848. [Google Scholar] [CrossRef]
- Das, D.; Kurungot, S. Porphyrin-Based Conducting Polymer Hydrogel for Supercapacitor Application. Energy Technol. 2020, 8, 2000061. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, F.; Dong, Z.H.; Shi, W. Synthesis of polypyrrole wrapped graphene hydrogels composites as supercapacitor electrodes. Electrochim. Acta 2013, 114, 125–132. [Google Scholar] [CrossRef]
- Zang, L.; Liu, Q.; Qiu, J.; Yang, C.; Wei, C.; Liu, C.; Lao, L. Design and fabrication of an all-solid-state polymer supercapacitor with highly mechanical flexibility based on polypyrrole hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 33941–33947. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, C.; Zhang, Z.; Zhong, W. Synthesis and Enhancement of Electroactive Biomass/Polypyrrole Hydrogels for High Performance Flexible All-Solid-State Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1901393. [Google Scholar] [CrossRef]
- Bo, J.; Luo, X.; Huang, H.; Li, L.; Lai, W.; Yu, X. Morphology-controlled fabrication of polypyrrole hydrogel for solid-state supercapacitor. J. Power Source 2018, 407, 105–111. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.; Simon, P.; Fauvarque, J.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Source 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Yang, C.-S.; Jang, Y.S.; Jeong, H.K. Bamboo-based activated carbon for supercapacitor applications. CAP 2014, 14, 1616–1620. [Google Scholar] [CrossRef]
- Gebeyehu, E.K.; Sui, X.; Adamu, B.F.; Beyene, K.A.; Tadesse, M.G. Cellulosic-Based Conductive Hydrogels for Electro-Active Tissues: A Review Summary. Gels 2022, 8, 140. [Google Scholar] [CrossRef]
- Carvalho, J.T.; Cunha, I.; Coelho, J.; Fortunato, E.; Martins, R.; Pereira, L. Carbon-Yarn-Based Supercapacitors with In Situ Regenerated Cellulose Hydrogel for Sustainable Wearable Electronics. ACS Appl. Energy Mater. 2022, 5, 11987–11996. [Google Scholar] [CrossRef]
- Ke, S.; Wang, Z.; Zhang, K.; Cheng, F.; Sun, J.; Wang, N.; Zhu, Y. Flexible conductive cellulose network-based composite hydrogel for multifunctional supercapacitors. Polymers 2020, 12, 1369. [Google Scholar] [CrossRef]
- Chen, S.-M.; Ramachandran, R.; Mani, V.; Saraswathi, R. Recent advancements in electrode materials for the high-performance electrochemical supercapacitors: A review. Int. J. Electrochem. Sci 2014, 9, 4072–4085. [Google Scholar]
- Liu, C.; Li, Y.; Zhuang, J.; Xiang, Z.; Jiang, W.; He, S.; Xiao, H. Conductive Hydrogels Based on Industrial Lignin: Opportunities and Challenges. Polymers 2022, 14, 3739. [Google Scholar] [CrossRef]
- Wang, D.; Yang, F.; Cong, L.; Feng, W.; Wang, C.; Chu, F.; Nan, J.; Chen, R. Lignin-containing hydrogel matrices with enhanced adhesion and toughness for all-hydrogel supercapacitors. Chem. Eng. J. 2022, 450, 138025. [Google Scholar] [CrossRef]
- Wan, H.; Qin, C.; Lu, A. A flexible, robust cellulose/phytic acid/polyaniline hydrogel for all-in-one supercapacitors and strain sensors. J. Mater. Chem. A 2022, 10, 17279–17287. [Google Scholar] [CrossRef]
- Kasaw, E.; Haile, A.; Getnet, M. Conductive coatings of cotton fabric consisting of carbonized charcoal for E-Textile. Coatings 2020, 10, 579. [Google Scholar] [CrossRef]
- Ahmadi, F.; Oveisi, Z.; Samani, S.M.; Amoozgar, Z. Chitosan based hydrogels: Characteristics and pharmaceutical applications. Res. Pharm. Sci. 2015, 10, 1. [Google Scholar] [PubMed]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for developing physical gels of chitosan and of chitosan derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Yang, M.; Wu, D.; Lyu, F.; Sun, Z.; Zhong, X.; Pan, H.; Liu, H.; Lu, Z. Biopolymer-chitosan based supramolecular hydrogels as solid state electrolytes for electrochemical energy storage. Chem. Commun. 2017, 53, 1615–1618. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wan, L.; Li, W.; Tan, X.; Du, X.; Tong, Y. A Self-Healing Gel Polymer Electrolyte, Based on a Macromolecule Cross-Linked Chitosan for Flexible Supercapacitors. Gels 2023, 9, 8. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Kong, L.; Kang, L.; Ran, F. Biopolymer-based carboxylated chitosan hydrogel film crosslinked by HCl as gel polymer electrolyte for all-solid-sate supercapacitors. J. Power Source 2019, 426, 47–54. [Google Scholar] [CrossRef]
- Yang, H.; Ji, X.; Tan, Y.; Liu, Y.; Ran, F. Modified supramolecular carboxylated chitosan as hydrogel electrolyte for quasi-solid-state supercapacitors. J. Power Source 2019, 441, 227174. [Google Scholar] [CrossRef]
- Selvam, S.; Yim, J.-H. Leakage free electrolyte engraved flexible supercapacitors from Chitosan/GO@ MnCO3 polymer hydrogel chelate film under BMIMBF4 ionic liquid assistance. J. Energy Storage 2021, 43, 103300. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Wang, S.; Wang, D.; Liu, F.; Cui, Y.; Liang, D.; Wang, X.; Yong, Z.; Chi, Y. Polyvinyl alcohol/quaternary ammonium chitosan hydrogel electrolyte for sensing supercapacitors with excellent performance. J. Energy Storage 2022, 46, 103918. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef]
- Yin, J.; Liu, Q.; Zhou, J.; Zhang, L.; Zhang, Q.; Rao, R.; Liu, S.; Jiao, T. Self-assembled functional components-doped conductive polypyrrole composite hydrogels with enhanced electrochemical performances. RSC Adv. 2020, 10, 10546–10551. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Al Kiey, S.A.; Darwish, A.; Turky, G.; Kamel, S. High performance hydrogel electrodes based on sodium alginate-g-poly (AM-c o-ECA-co-AMPS for supercapacitor application. Int. J. Biol. Macromol. 2022, 218, 420–430. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Stojkovska, J.; Obradović, B.; Mišković-Stanković, V. Alginate hydrogel microbeads incorporated with Ag nanoparticles obtained by electrochemical method. Mater. Chem. Phys. 2012, 133, 182–189. [Google Scholar] [CrossRef]
- Peng, K.; Wang, W.; Zhang, J.; Ma, Y.; Lin, L.; Gan, Q.; Chen, Y.; Feng, C. Preparation of Chitosan/Sodium alginate conductive hydrogels with high salt contents and their application in flexible supercapacitors. Carbohydr. Polym. 2022, 278, 118927. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Gong, Q.; Xia, Z.; Yang, Y.; Chen, C.; Qian, C. Facile preparation of stretchable and self-healable conductive hydrogels based on sodium alginate/polypyrrole nanofibers for use in flexible supercapacitor and strain sensors. Int. J. Biol. Macromol. 2021, 172, 41–54. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Yin, J.; Wei, K.; Zhang, J.; Liu, S.; Wang, X.; Wang, X.; Zhang, Q.; Qin, Z.; Jiao, T. MXene-based film electrode and all-round hydrogel electrolyte for flexible all-solid supercapacitor with extremely low working temperature. Cell Rep. Phys. Sci. 2022, 23, 4248–4253. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Liu, Y.; Liang, D. Nanocellulose-Linked MXene/Polyaniline Aerogel Films for Flexible Supercapacitors. Gels 2022, 8, 798. [Google Scholar] [CrossRef]
- Dutta, P.; Sikdar, A.; Majumdar, A.; Borah, M.; Padma, N.; Ghosh, S.; Maiti, U.N. Graphene aided gelation of MXene with oxidation protected surface for supercapacitor electrodes with excellent gravimetric performance. Carbon 2020, 169, 225–234. [Google Scholar] [CrossRef]
- Ma, C.; Ma, M.G.; Si, C.; Ji, X.X.; Wan, P. Flexible MXene-based composites for wearable devices. Adv. Funct. Mater. 2021, 31, 2009524. [Google Scholar] [CrossRef]
- Ren, M.; Di, J.; Chen, W. Recent progress and application challenges of wearable supercapacitors. Batter. Supercaps 2021, 4, 1279–1290. [Google Scholar] [CrossRef]
- Lu, C.; Chen, X. All-temperature flexible supercapacitors enabled by antifreezing and thermally stable hydrogel electrolyte. Nano Lett. 2020, 20, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Armelin, E.; Pérez-Madrigal, M.M.; Alemán, C.; Díaz, D.D. Current status and challenges of biohydrogels for applications as supercapacitors and secondary batteries. J. Mater. Chem. A 2016, 4, 8952–8968. [Google Scholar] [CrossRef] [Green Version]









| Composite Materials | Methods | Capacitance Value | Ref. |
|---|---|---|---|
| Hydrazine hydrate | Chemical oxidation | 190 F/g at 0.5 A/g | [48] |
| Chitosan | Microwave-assisted hydrothermal | 377 F/g at 5 A/g | [51] |
| Chitosan | One-step hydrothermal method | 375.7 F/g at 1 A/g | [52] |
| Ethylenediamine | Two-step hydrothermal method | −240 F/g at 1 A/g | [45] |
| Carbon nanotube | Chemical reduction | 10.13 mF/cm2 | [53] |
| PVA/Zn/EG | Chemical dissolution | 247.7 F/g at 5 A/g | [54] |
| PVA | Microwave-assisted cross-linking | 163 F/g at 1 A/g | [55] |
| Potassium acetate | Hydrothermal | 55.1 F/g at 0.3 A/g | [56] |
| Polyaniline | Polymerization | 500.13 mF cm−2 | [57] |
| Chitosan | In situ ion cross linking | 107.6 F/g at 1 A/g | [58] |
| CuS/ZnS/sodium alginate | Physical crosslinking followed by one-step reduction | 252.1 F/g at 5 mV/s | [59] |
| Ti3C2Tx MXene | Gelation process | 26 F/g at 1 V/s | [60] |
| NiCo oxide | Coagulation-induced self-assembly | 858.3 F/g at 2 A/g | [61] |
| CoFe2O4 | In situ via a facile one-pot solvothermal approach | 356 F/g at 0.5 A/g | [62] |
| Polyampholyte | One-step random copolymerization | 216 F/g at 0.5 A/g | [63] |
| β-cyclodextrin | Hydrothermal reduction | 310.8 F/g at 0.5 A/g | [64] |
| Composite Materials | Methods | Capacitance Value | Ref. |
|---|---|---|---|
| Cellulose | Induced polymerization and cross-linking | 1786 mF/cm2 at 1 mA/cm2 | [67] |
| Graphene | Aerosol chemical vapor deposition | 806 F cm−3 at 112 mWcm−3 | [68] |
| ZnNiCo hydroxide/graphene | Catalytic carbon vapor deposition | 1.185 mAh cm−2 at 5 mA cm−2 | [69] |
| Nanocellulose/PVA | Dissolution | 117.1 F g−1 at 0.3 A/g | [70] |
| Nanocellulose | Oxidation | 65 F g−1 at 0.4 A/g | [71] |
| Polyaniline | Graft polymerization | 880 F g−1 at 1.5 A/g | [72] |
| Carboxymethylcellulose-polyaniline | Layer-by-layer assembly | 3106.3 mF cm−2 at 5 mA cm−2 | [73] |
| Polyaniline | In situ chemical oxidation | 647 F g−1 at 1 A/g | [74] |
| Composite Materials | Methods | Capacitance Value | Ref. |
|---|---|---|---|
| Polyvinyl alcohol (PVA) | Solution immersion | 128.9 mF cm−2 at 11.46 µWh cm−2 | [94] |
| Bis(trifluoromethane) sulfonamide lithium Salt (LiTFSI), PVA | Novel incorporation | 44.5 mF cm−2 at 0.04 mW cm−2 | [95] |
| Molybdenum disulfide | Hydrothermal process | 360 mF cm–2 at 0.5 mA cm−2 | [96] |
| Aluminiumchlorid | Induced cross-linking | 158 F/g at 0.6 A/g | [97] |
| Poly (acrylamide) | Free radical polymerization | 327 F/g at 1 A/g | [98] |
| PVA/poly (methacrylic acid) | Polymerization | 7.38 mF cm−2 at 10 mA cm−2 | [99] |
| H2SO4 | Thermal treatment | 202 F cm−3 at 0.54 A cm−3 | [100] |
| Polyaniline | Polymerization | 112.6 F/g at 0.25 mWh cm−3 | [101] |
| Polyaniline | Thermal process | 808.2 mF cm−2 at 0.63 mWh cm−3 | [102] |
| MWCNT | Thermal treatment | 485 F/g at 1 A/g | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadesse, M.G.; Lübben, J.F. Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels 2023, 9, 106. https://doi.org/10.3390/gels9020106
Tadesse MG, Lübben JF. Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels. 2023; 9(2):106. https://doi.org/10.3390/gels9020106
Chicago/Turabian StyleTadesse, Melkie Getnet, and Jörn Felix Lübben. 2023. "Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications" Gels 9, no. 2: 106. https://doi.org/10.3390/gels9020106
APA StyleTadesse, M. G., & Lübben, J. F. (2023). Review on Hydrogel-Based Flexible Supercapacitors for Wearable Applications. Gels, 9(2), 106. https://doi.org/10.3390/gels9020106