Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Niosomes Preparation
2.3. Niosomes Characterization
2.4. AMs Content in Niosomes
2.5. In Vitro Diffusion Experiments
2.6. Antioxidant Activity
2.7. Niosomal Gel Preparation and Characterization
2.8. Patch Test
3. Results and Discussion
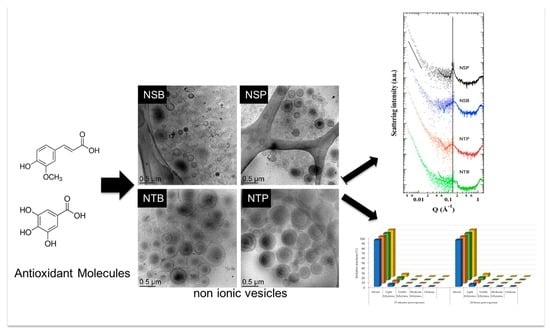
3.1. Production and Characterization of AMs-Loaded Niosomes
3.2. Encapsulation Efficiency of Ams
3.3. In Vitro Diffusion Kinetics
3.4. Antioxidant Activity
3.5. Niosomal Gel Production and Technological Behavior
3.6. AMs Diffusion from Niosomal Gels
3.7. Patch Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dizdaroglu, M.; Jaruga, P.; Birincioglu, M.; Rodriguez, H. Free Radical-Induced Damage to DNA: Mechanisms and Measurement. Free. Radic. Biol. Med. 2002, 32, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for Delivery of Antioxidants in Cosmeceuticals: Challenges and Development Strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Esposito, E.; Cortesi, R. Lipid-Based Nanosystems as a Tool to Overcome Skin Barrier. Int. J. Mol. Sci. 2021, 22, 8319. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Jeon, B. Synergistic Anti-Campylobacter Jejuni Activity of Fluoroquinolone and Macrolide Antibiotics with Phenolic Compounds. J. Antibiot. 2015, 6, 591–593. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Li, J.; Li, J.; Tang, R.; Liu, L.; Shi, J.; Huang, Q.; Yang, H. Inhibition of Gallic Acid on the Growth and Biofilm Formation of Escherichia coli and Streptococcus mutans. J. Food Sci. 2015, 80, M1299–M1305. [Google Scholar] [CrossRef]
- Sorrentino, E.; Succi, M.; Tipaldi, L.; Pannella, G.; Maiuro, L.; Sturchio, M.; Coppola, R.; Tremonte, P. Antimicrobial Activity of Gallic Acid against Food-Related Pseudomonas Strains and Its Use as Biocontrol Tool to Improve the Shelf Life of Fresh Black Truffles. Int. J. Food Microbiol. 2018, 266, 183–189. [Google Scholar] [CrossRef]
- Fu, R.; Zhang, Y.; Peng, T.; Guo, Y.; Chen, F. Phenolic Composition and Effects on Allergic Contact Dermatitis of Phenolic Extracts Sapium sebiferum (L.) Roxb. Leaves. J. Ethnopharmacol. 2015, 162, 176–180. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Q.; Huang, X.; Jiang, G.; Li, C.; Zhang, X.; Liu, S.; He, L.; Liu, Y.; Dai, Q.; et al. Effects of Dietary Ferulic Acid on the Intestinal Microbiota and the Associated Changes on the Growth Performance, Serum Cytokine Profile, and Intestinal Morphology in Ducks. Front. Microbiol. 2021, 12, 698213. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F.; Rizza, L.; Cortesi, R.; Merlotti, E.; Drechsler, M.; Mariani, P.; Contado, C.; Ravani, L.; Esposito, E. Evaluation of Percutaneous Absorption of Naproxen from Different Liposomal Formulations. J. Pharm. Sci. 2010, 99, 2819–2829. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, L.R.S.; Ortore, M.G.; Spinozzi, F.; Mariani, P.; Bernstorff, S.; Itri, R. The Importance of Protein-Protein Interactions on the PH-Induced Conformational Changes of Bovine Serum Albumin: A Small-Angle X-Ray Scattering Study. Biophys. J. 2010, 98, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Pecora, R. Dynamic Light Scattering Measurement of Nanometer Particles in Liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
- Andrade, L.M.; de Fátima Reis, C.; Maione-Silva, L.; Anjos, J.L.V.; Alonso, A.; Serpa, R.C.; Marreto, R.N.; Lima, E.M.; Taveira, S.F. Impact of Lipid Dynamic Behavior on Physical Stability, In Vitro Release and Skin Permeation of Genistein-Loaded Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 40–47. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Rangarajan, M.; Shao, Y.; LaVoie, E.J.; Huang, T.-C.; Ho, C.-T. Antioxidative Phenolic Compounds from Sage (Salvia officinalis). Korean J. Pestic. Sci. 1998, 46, 4869–4873. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Panico, A.M.; Cardile, V.; Crascì, L.; Carducci, F.; Graziano, A.C.E.; Cortesi, R.; Puglia, C. Nanostructured Lipid Dispersions for Topical Administration of Crocin, a Potent Antioxidant from Saffron (Crocus sativus L.). Mater. Sci. Eng. C 2017, 71, 669–677. [Google Scholar] [CrossRef]
- Robinson, M.K.; Cohen, C.; de Brugerolle de Fraissinette, A.; Ponec, M.; Whittle, E.; Fentem, J.H. Non-Animal Testing Strategies for Assessment of the Skin Corrosion and Skin Irritation Potential of Ingredients and Finished Products. Food Chem. Toxicol. 2002, 40, 573–592. [Google Scholar] [CrossRef] [PubMed]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety Assessment of Personal Care Products/Cosmetics and Their Ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef] [PubMed]
- The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Opinion Concerning Basic Criteria of the Protocols for the Skin Compatibility Testing of Potentially Cutaneous Irritant Cosmetic Ingredients or Mixtures of Ingredients on Human Volunteers; SCCNFP/0245/99; SCCNFP: Brussels, Belgium, 1999. [Google Scholar]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic Acid: A Versatile Antioxidant with Promising Therapeutic and Industrial Applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.-C. Ferulic Acid: Pharmaceutical Functions, Preparation and Applications in Foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Franzè, S.; Musazzi, U.M.; Minghetti, P.; Cilurzo, F. Drug-in-Micelles-in-Liposomes (DiMiL) Systems as a Novel Approach to Prevent Drug Leakage from Deformable Liposomes. Eur. J. Pharm. Sci. 2019, 130, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, P.; Funari, S.S.; La Mesa, C.; Mariani, P.; Ortore, M.G.; Sinibaldi, R.; Spinozzi, F. Multi- to Unilamellar Transitions in Catanionic Vesicles. J. Phys. Chem. B 2010, 114, 8056–8060. [Google Scholar] [CrossRef] [Green Version]
- Rappolt, M.; Gregorio, G.M.D.; Almgren, M.; Amenitsch, H.; Pabst, G.; Laggner, P.; Mariani, P. Non-Equilibrium Formation of the Cubic Pn 3 m Phase in a Monoolein/Water System. Europhys. Lett. 2006, 75, 267–273. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Mahale, N.B.; Thakkar, P.D.; Mali, R.G.; Walunj, D.R.; Chaudhari, S.R. Niosomes: Novel Sustained Release Nonionic Stable Vesicular Systems—An Overview. Adv. Colloid Interface Sci. 2012, 183–184, 46–54. [Google Scholar] [CrossRef]
- Sguizzato, M.; Valacchi, G.; Pecorelli, A.; Boldrini, P.; Simelière, F.; Huang, N.; Cortesi, R.; Esposito, E. Gallic Acid Loaded Poloxamer Gel as New Adjuvant Strategy for Melanoma: A Preliminary Study. Colloids Surf. B Biointerfaces 2020, 185, 110613. [Google Scholar] [CrossRef]
- Marino, T.; Galano, A.; Russo, N. Radical Scavenging Ability of Gallic Acid toward OH and OOH Radicals. Reaction Mechanism and Rate Constants from the Density Functional Theory. J. Phys. Chem. B 2014, 118, 10380–10389. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, J.; Hao, Y.; Liu, Y. Identification of the DPPH Radical Scavenging Reaction Adducts of Ferulic Acid and Sinapic Acid and Their Structure-Antioxidant Activity Relationship. LWT 2021, 146, 111411. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Ma, H.; Yuan, E.; Li, Q. Characterization and DPPH Radical Scavenging Activity of Gallic Acid-Lecithin Complex. Trop. J. Pharm. Res. 2014, 13, 1333. [Google Scholar] [CrossRef] [Green Version]
- Witczak, Z.J. Polysaccharides in Medicinal Applications. Edited by Severian Dumitriu, Marcel Dekker, Inc. New York, ISBN 0-8247-9540-7. 1996, 794 pp. $195.00. J. Carbohydr. Chem. 1997, 16, 245–247. [Google Scholar] [CrossRef]
- Minnelli, C.; Moretti, P.; Fulgenzi, G.; Mariani, P.; Laudadio, E.; Armeni, T.; Galeazzi, R.; Mobbili, G. A Poloxamer-407 Modified Liposome Encapsulating Epigallocatechin-3-Gallate in the Presence of Magnesium: Characterization and Protective Effect against Oxidative Damage. Int. J. Pharm. 2018, 552, 225–234. [Google Scholar] [CrossRef]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin Delivery of Epigallocatechin-3-Gallate (EGCG) and Hyaluronic Acid Loaded Nano-Transfersomes for Antioxidant and Anti-Aging Effects in UV Radiation Induced Skin Damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Xiao, H.; Zhao, J.; Zhao, T. Cardioprotective Effect of Sodium Ferulate in Diabetic Rats. Int. J. Med. Sci. 2012, 9, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Karamać, M.; Koleva, L.; Kancheva, V.; Amarowicz, R. The Structure–Antioxidant Activity Relationship of Ferulates. Molecules 2017, 22, 527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, X.; Huang, S.; Deng, M.; Xie, X.; Liu, M.; Liu, H.; Zhou, X.; Li, J.; Ten Cate, J.M. Changes in Composition and Enamel Demineralization Inhibition Activities of Gallic Acid at Different PH Values. Acta Odontol. Scand. 2015, 73, 595–601. [Google Scholar] [CrossRef]







| Acronym | Composition | |||||
|---|---|---|---|---|---|---|
| Molar Ratio | Aqueous Phase | Organic Phase (mg/mL) | AM (mg/mL) | |||
| Cholesterol | Span 20 | Tween 20 | ||||
| GA/B; FA/B | - | - | - | Borate Buffer (B) | - | 2 |
| GA/P; FA/P | - | - | - | Poloxamer 188 * (P) | - | 2 |
| NSB-GA; NSB-FA | 1 | 1 | - | Borate Buffer (B) | 25 | 2 |
| NSP-GA; NSP-FA | 1 | 1 | - | Poloxamer 188 * (P) | 25 | 2 |
| NTB-GA; NTB-FA | 1 | - | 1 | Borate Buffer (B) | 25 | 2 |
| NTP-GA; NTP-FA | 1 | - | 1 | Poloxamer 188 * (P) | 25 | 2 |
| Niosome Acronym | Peak Position (2θ) | Peak Width (2θ) | Unit Cell d (nm) | Mean Crystallite Size L (nm) | Approximate Number of Interacting Bilayer L/d |
|---|---|---|---|---|---|
| NSP | 1.299 | 0.129 | 4.41 | 41.7 | 9 |
| NSB | 1.290 | 0.554 | 4.44 | 9.7 | 2 |
| NTP | 1.180 | 0.388 | 4.86 | 13.9 | 3 |
| NTB | 1.191 | 0.681 | 4.81 | 7.9 | 2 |
| errors | ± 0.10 | ± 20% |
| Time (d) | NSB-GA | NSP-GA | NTB-GA | NTP-GA | NSB-FA | NSP-FA | NTB-FA | NTP-FA |
|---|---|---|---|---|---|---|---|---|
| Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | Z-Ave (nm) | |
| PdI | PdI | PdI | PdI | PdI | PdI | PdI | PdI | |
| 1 | 549 ± 49 | 456 ± 28 | 436 ± 39 | 610 ± 16 | 419 ± 61 | 594 ± 4 | 495 ± 41 | 862 ± 53 |
| 0.13 ± 0.07 | 0.19 ± 0.07 | 0.15 ± 0.03 | 0.25 ± 0.01 | 0.27 ± 0.10 | 0.36 ± 0.02 | 0.27 ± 0.03 | 0.39 ± 0.05 | |
| 7 | 614 ± 35 | 573 ± 35 | 451 ± 32 | 686 ± 31 | 373 ± 21 | 499 ± 39 | 473 ± 42 | 895 ± 27 |
| 0.11 ± 0.02 | 0.30 ± 0.03 | 0.26 ± 0.10 | 0.40 ± 0.05 | 0.31 ± 0.07 | 0.27 ± 0.03 | 0.28 ± 0.01 | 0.35 ± 0.03 | |
| 15 | 613 ± 50 | 604 ± 44 | 501 ± 14 | 825 ± 26 | 377 ± 25 | 456 ± 38 | 498 ± 34 | 932 ± 31 |
| 0.17 ± 0.03 | 0.24 ± 0.04 | 0.41 ± 0.03 | 0.28 ± 0.09 | 0.22 ± 0.09 | 0.23 ± 0.08 | 0.30 ± 0.01 | 0.33 ± 0.08 | |
| 30 | 601 ± 46 | 779 ± 37 | 546 ± 28 | 1021 ± 48 | 479 ± 29 | 422 ± 26 | 526 ± 23 | 1082 ± 98 |
| 0.21 ± 0.01 | 0.26 ± 0.02 | 0.31 ± 0.02 | 0.29 ± 0.07 | 0.25 ± 0.03 | 0.24 ± 0.01 | 0.29 ± 0.02 | 0.32 ± 0.15 |
| Acronym | Spreadability (g·cm/s) | Leakage (s) |
|---|---|---|
| xg-NSB | 3.10 ± 0.25 | n.d. |
| xg-NSP | 1.52 ± 0.09 | n.d. |
| xg-NTB | 3.02 ± 0.19 | n.d. |
| xg-NTP | 1.03 ± 0.07 | n.d. |
| pol-NSB | 8.93 ± 0.52 | 8.49 ± 0.38 |
| pol-NSP | 4.18 ± 0.29 | 7.16 ± 0.42 |
| pol-NTB | 9.77 ± 0.64 | 8.23 ± 0.48 |
| pol-NTP | 4.08 ± 0.22 | 7.36 ± 0.32 |
| Acronym | pH | ||
|---|---|---|---|
| Niosomes 1 | xg-Niosomes 2 | pol-Niosomes 3 | |
| NSB-GA | 4.7 ± 0.5 | 5.2 ± 0.3 | 5.4 ± 0.1 |
| NSP-GA | 4.9 ± 0.1 | 4.8 ± 0.1 | 4.9 ± 0.2 |
| NTB-GA | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.9 ± 0.1 |
| NTP-GA | 4.7 ± 0.1 | 4.9 ± 0.2 | 5.4 ± 0.2 |
| NSB-FA | 6.0 ± 0.3 | 6.3 ± 0.3 | 6.5 ± 0.1 |
| NSP-FA | 5.2 ± 0.3 | 5.1 ± 0.2 | 5.6 ± 0.1 |
| NTB-FA | 6.0 ± 0.1 | 5.9 ± 0.2 | 6.0 ± 0.2 |
| NTP-FA | 5.5 ± 0.2 | 5.4 ± 0.2 | 5.3 ± 0.2 |
| Acronym | GA | FA | ||
|---|---|---|---|---|
| Js (μg/cm2·h) | Jn (cm2·h) | Js (μg/cm2·h) | Jn (cm2·h) | |
| NSB | 81.0 | 40.5 | 176.7 | 88.3 |
| xg-NSB | 66.0 | 33.0 | 58.9 | 29.4 |
| pol-NSB | 120.0 | 60.0 | 78.2 | 39.1 |
| NSP | 45.3 | 22.7 | 83.3 | 41.6 |
| xg-NSP | 22.6 | 11.3 | 34.1 | 17.0 |
| pol-NSP | 95.7 | 47.8 | 43.8 | 21.9 |
| NTB | 220.7 | 110.4 | 119.5 | 59.8 |
| xg-NTB | 53.1 | 26.6 | 114.4 | 57.2 |
| pol-NTB | 119.2 | 59.6 | 112.1 | 56.1 |
| NTP | 70.3 | 35.1 | 82.4 | 41.2 |
| xg-NTP | 36.2 | 18.1 | 104.5 | 52.3 |
| pol-NTP | 112.9 | 56.5 | 92.3 | 46.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels 2023, 9, 107. https://doi.org/10.3390/gels9020107
Sguizzato M, Pepe A, Baldisserotto A, Barbari R, Montesi L, Drechsler M, Mariani P, Cortesi R. Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels. 2023; 9(2):107. https://doi.org/10.3390/gels9020107
Chicago/Turabian StyleSguizzato, Maddalena, Alessia Pepe, Anna Baldisserotto, Riccardo Barbari, Leda Montesi, Markus Drechsler, Paolo Mariani, and Rita Cortesi. 2023. "Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior" Gels 9, no. 2: 107. https://doi.org/10.3390/gels9020107
APA StyleSguizzato, M., Pepe, A., Baldisserotto, A., Barbari, R., Montesi, L., Drechsler, M., Mariani, P., & Cortesi, R. (2023). Niosomes for Topical Application of Antioxidant Molecules: Design and In Vitro Behavior. Gels, 9(2), 107. https://doi.org/10.3390/gels9020107