Biomaterials Based on Chitosan and Polyvinyl Alcohol as a Drug Delivery System with Wound-Healing Effects
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Spectroscopy
2.2. Morphological Characterization via Scanning Electron Microscopy (SEM)
2.3. Dynamic Water Vapor Sorption Capacity
2.4. Estimation of Drug Loading and Entrapment Efficiency
2.5. In Vitro Drug Release and Permeation Studies
2.6. Analysis of In Vitro Drugs Release Kinetics
3. Conclusions
4. Materials and Methods
4.1. Reagents
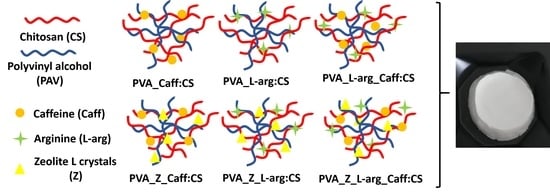
4.2. Preparation of the Samples
4.3. Methods
4.3.1. Attenuated Total Reflection Fourier Transform IR (ATR-FTIR) Spectroscopy
4.3.2. Morphological Characterization Via Scanning Electron Microscopy (SEM)
4.3.3. Dynamic Water Vapor Sorption Capacity
4.3.4. Estimation of Drug Loading and Entrapment Efficiency
4.3.5. In Vitro Drug Release and Permeation Studies
4.3.6. Analysis of In Vitro Drugs Release Kinetics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tavakoli, S.; Klar, A.S. Bioengineered skin substitutes: Advances and future trends. Appl. Sci. 2021, 11, 1493. [Google Scholar] [CrossRef]
- Urciuolo, F.; Casale, C.; Imparato, G.A.; Netti, P.A. Bioengineered skin substitutes: The role of extracellular matrix and vascularization in the healing of deep wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, R.V.; James, S.L.; James, S.E. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J. R. Soc. Interface 2010, 7, 229–258. [Google Scholar] [CrossRef]
- Selvan, N.K.; Shanmugarajan, T.S.; Uppuluri, V.N.V.A. Hydrogel based scaffolding polymeric biomaterials: Approaches towardsskin tissue regeneration. J. Drug Deliv. Sci. Technol. 2020, 55, 101456. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Ostrózka-Cieślik, A. The potential of pharmaceutical hydrogels in the formulation of topical administration hormone drugs. Polymers 2022, 14, 3307. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Sobczak, M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics 2020, 12, 396. [Google Scholar] [CrossRef]
- Stan, D.; Tanase, C.; Avram, M.; Apetrei, R.; Mincu, N.B.; Mateescu, A.L.; Stan, D. Wound healing applications of creams and “smart” hydrogels. Exp. Dermatol. 2021, 30, 1218–1232. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. In Biopolymers PVA Hydrogels Anionic Polymerisation Nanocomposites; Abe, A., Albertsson, A.C., Cantow, H.J., Dusek, K., Edwards, S., Höcker, H., Joanny, J.F., Kausch, H.H., Kobayashi, T., Lee, K.S., et al., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2000; Volume 153, pp. 37–65. [Google Scholar]
- Yang, W.; Fortunatia, E.; Bertoglioc, F.; Owczarekb, J.S.; Brunif, G.; Kozaneckib, M.; Kennya, J.M.; Torrea, L.; Visaic, L.; Pugliaa, D. Polyvinyl alcohol/chitosan hydrogels with enhanced antioxidant and antibacterial properties induced by lignin nanoparticles. Carbohydr. Polym. 2018, 181, 275–284. [Google Scholar] [CrossRef]
- Muppalaneni, S.; Omidian, H. Polyvinyl alcohol in medicine and pharmacy: A perspective. J. Develop. Drugs 2013, 2, 1000112. [Google Scholar] [CrossRef] [Green Version]
- Păduraru, O.M.; Ciolacu, D.; Darie, R.N.; Vasile, C. Synthesis and characterization of polyvinyl alcohol/cellulose cryogels and their testing as carriers for a bioactive component. Mater. Sci. Eng. C 2012, 32, 2508–2515. [Google Scholar] [CrossRef]
- Baharlouei, P.; Rahman, A. Chitin and chitosan: Prospective biomedical applications in drug delivery, cancer treatment, and wound healing. Mar. Drugs 2022, 20, 460. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Shitiz, K.; Singh, A. Chitin and chitosan: Biopolymers for wound management. Int. Wound J 2017, 14, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Jin, S.G. Production and application of biomaterials based on polyvinyl alcohol (PVA) as wound dressing. Chem. Asian J. 2022, 17, e202200595. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly(vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- Wan, W.; Bannerman, A.D.; Yang, L.; Mak, H. Poly(vinyl alcohol) cryogels for biomedical applications. In Polymeric Cryogels: Macroporous Gels with Remarkable Properties; Okay, O., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2014; Volume 263, pp. 283–321. [Google Scholar]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Nechita, P. Applications of chitosan in wastewater treatment. In Biological Activities and Application of Marine Polysaccharides; Shalaby, E.A., Ed.; Intech Open: London, UK, 2017; pp. 209–228. [Google Scholar]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The multifunctional role of chitosan in horticultural crops; A review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of chitin/chitosan and their derivatives in the papermaking industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Llanos Gandía, M.; Caballero, A.H. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Notario-Pérez, F.; Martín-Illana, A.; Cazorla-Luna, R.; Ruiz-Caro, R.; Veiga, M.D. Applications of chitosan in surgical and post-surgical materials. Mar. Drugs 2022, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadi, A.; Ansari, M.J.; Deepak, A. Recent advances of chitosan formulations in biomedical applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J.; et al. Temperature- and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef]
- Kong, X.; Xu, W.; Zhang, C.; Kong, W. Chitosan temperature sensitive gel loaded with drug microspheres has excellent effectiveness, biocompatibility and safety as an ophthalmic drug delivery system. Exp. Ther. Med. 2018, 15, 1442–1448. [Google Scholar] [CrossRef]
- Figueroa-Pizano, M.D.; Vélaz, I.; Martínez-Barbosa, M.E. A freeze-thawing method to prepare chitosan-poly(vinyl alcohol) hydrogels without crosslinking agents and diflunisal release studies. J. Vis. Exp. 2020, 155, e59636. [Google Scholar]
- Prabowo, W.H.; Prasetyo, A.; Susilaningsih, N. The effect of multilevel doses of caffeine on tissue macrophage and blood lymphocyte count in autologous full thickness skin graft healing in sprague dawley rats. Biosci. Med. J. Biomed. Transl. Res. 2022, 5, 1697–1702. [Google Scholar] [CrossRef]
- Bonyanian, Z.; Rose’Meyer, R.B. Caffeine and its potential role in attenuating impaired wound healing in diabetes. J. Caffeine Res. 2015, 5, 141–148. [Google Scholar] [CrossRef]
- Nicoli, S.; Colombo, P.; Santi, P. Release and permeation kinetics of caffeine from bioadhesive transdermal films. AAPS J. 2005, 7, E218–E223. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Huang, H.; Wu, J. Advances and impact of arginine-based materials in wound healing. J. Mater. Chem. B 2021, 9, 6738–6750. [Google Scholar] [CrossRef] [PubMed]
- Iacob, A.T.; Drăgan, M.; Ghetu, N.; Pieptu, D.; Vasile, C.; Buron, F.; Routier, S.; Giusca, S.E.; Caruntu, I.D.; Profire, L. Preparation, characterization and wound healing effects of new membranes based on chitosan, hyaluronic acid and arginine derivatives. Polymers 2018, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- El-Say, K.M. Maximizing the encapsulation efficiency and the bioavailability of controlled-release cetirizine microspheres using Draper–Lin small composite design. Drug Des. Devel. Ther. 2016, 10, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Deng, J.; Zhang, Z.; Sui, H.; Shi, W.; Yuan, B.; Lin, H.; Yang, X.; Cao, J.; Zhu, X.; et al. Spatiotemporal manipulation of L-arginine release from bioactive hydrogels initiates rapid skin wound healing accompanied with repressed scar formation. Appl. Mater. Today 2021, 24, 101116. [Google Scholar] [CrossRef]
- Kumar, S.; Rai, S.B. Spectroscopic studies of L-arginine molecule. Ind. J. Pure Appl. Phys. 2010, 48, 251–255. [Google Scholar]
- Negrea, P.; Caunii, A.; Sarac, I.; Butnariu, M. The study of infrared spectrum of chitin and chitosan extract as potential sources of biomass. Dig. J. Nanomater. Biostruct. 2015, 10, 1129–1138. [Google Scholar]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS–PVA nanofluids synthesized through a radiolytic approach. J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Ipate, A.M.; Hamciuc, C.; Kalvachev, Y.; Gherman, S.; Ochiuz, L. New cryogels based on polymers and zeolite L for controlled Enalapril maleate release. J. Drug Deliv. Sci. Technol. 2018, 44, 505–512. [Google Scholar] [CrossRef]
- Gherman, S.; Zavastin, D.; Ochiuz, L.; Biliuta, G.; Coseri, S. Enalapril maleate loaded pullulan film for mucoadhesive buccal drug delivery applications. Cell Chem. Technol. 2016, 50, 593–600. [Google Scholar]
- Rajam, K.; Rajendran, S.; Banu, N.N. Effect of caffeine-Zn2+ system in preventing corrosion of carbon. J. Chem. 2013, 2013, 521951. [Google Scholar] [CrossRef]
- Oyebanji, J.A.; Okekunle, P.O.; Fayomi, O.S.I. Synthesis and characterization of zeolite-Y using Ficus exasperata leaf: A preliminary study. Case. Stud. Chem. Environ. Eng. 2020, 2, 100063. [Google Scholar] [CrossRef]
- Ahuja, G.; Pathak, K. Porous carriers for controlled/modulated drug delivery. Indian J. Pharm. Sci. 2009, 71, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.D.; Lemus, R.; Pérez, C.E. Models of sorption isotherms for food: Uses and limitations. Vitae Rev. De La Fac. De Química Farm. 2011, 18, 325–334. [Google Scholar] [CrossRef]
- Ipate, A.M.; Serbezeanu, D.; Bargan, A.; Hamciuc, C.; Ochiuz, L.; Gherman, S. Poly(vinylpyrrolidone)-chitosan hydrogels as matrices for controlled drug release. Cell Chem. Technol. 2021, 55, 63–73. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Hybrid super-porous hydrogel composites with high water vapor adsorption capacity—Adsorption isotherm and kinetics studies. J. Environ. Chem. Eng. 2021, 9, 106611. [Google Scholar] [CrossRef]
- Mittal, H.; Al Alili, A.; Alhassan, S.M. Solid polymer desiccants based on poly(acrylic acid-co-acrylamide) and Laponite RD: Adsorption isotherm and kinetics studies. Colloids Surf. A 2020, 599, 124813. [Google Scholar] [CrossRef]
- Aguirre Loredo, R.Y.; Velazquez, G.; Guadarrama Lezama, A.Y.; Viveros Contreras, R.; Castaño, J. Water adsorption thermodynamical analysis and mechanical characterization of chitosan and polyvinyl alcohol based films. J. Polym. Environ. 2022, 30, 1880–1892. [Google Scholar] [CrossRef]
- Li, X.; Chao, G.; Wang, L.; Xu, X.; Cai, Z.; Shi, L.; Zhuang, X.; Cheng, B. Preparation and BSA adsorption behavior of chitosan-arginine based nanofiber membranes. Fibers Polym. 2018, 19, 941–948. [Google Scholar] [CrossRef]
- Abdel Azeem, S.M.; Ali, S.; El-Shahat, M.F. Sorption characteristics of caffeine onto untreated polyurethane foam: Application to its determination in human plasma. Anal. Sci 2011, 27, 1133–1137. [Google Scholar] [CrossRef]
- Delgado, R. Misuse of Beer–Lambert Law and other calibration curves. R. Soc. Open Sci. 2021, 9, 211103. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Chen, T.; Bao, X. Zeolites: A series of promising biomaterials in bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1066552. [Google Scholar] [CrossRef]
- Murrieta-Ricoa, F.N.; Antúnez-Garcíab, J.; Yocupicio-Gaxiolac, R.I.; Galvánb, D.H.; González, J.C.; Petranovskii, V. Zeolites as initial structures for the preparation of functional materials. J. Appl. Res. Technol. 2022, 20, 92–116. [Google Scholar] [CrossRef]
- Yuwono, H.S. Why the coffee powder is the best topical wound dressing? EJMED 2021, 3, 4–7. [Google Scholar] [CrossRef]
- Heredia, N.S.; Vizuete, K.; Flores-Calero, M.; Pazmiño, K.V.; Pilaquinga, F.; Kumar, B.; Debut, A. Comparative statistical analysis of the release kinetics models for nanoprecipitated drug delivery systems based on poly (lactic-coglycolic acid). PLoS ONE 2022, 17, 0264825. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, C.; Ranetti, A.E.; Hinescu, L.G.; Ionescu, M.; Cosmescu, C.; Postoarca, A.G.; Cinteza, L.O. Formulation and evaluation of in vitro release kinetics of na3cadtpa decorporation agent embedded in microemulsion-based gel formulation for topical delivery. Farmacia 2015, 63, 656–664. [Google Scholar]
- Singhvi, G.; Singh, M. Review: In-vitro drug release characterization models. Int. J. Pharm. Stud. Res. 2011, II, 77–84. [Google Scholar]
- Kumar, P.; Ganure, A.L.; Subudhi, B.B.; Shukla, S. Design and comparative evaluation of in-vitro drug release, pharmacokinetics and gamma scintigraphic analysis of controlled release tablets using novel ph sensitive starch and modified starch-acrylate graft copolymer matrices. Iran J. Pharm. Res. 2015, 14, 677–691. [Google Scholar]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic Viscosity–Molecular Weight Relationship for Chitosan. J. Polym. Sci. B Polym. Phys. 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Sadegh Hassani, S.; Salehirad, F.; Aghabozorg, H.R.; Sobat, Z. Synthesis and morphology of nanosized zeolite L. Cryst. Res. Technol. 2010, 45, 183–187. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the freeze–thaw technique affect the properties of the alginate/chitosan glutamate gels with posaconazole as a model antifungal drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef]
- Clowes, H.M.; Scott, R.C.; Heylings, J.R. Skin absorption: Flow-through or static diffusion cells. Toxicol. Vitr. 1994, 8, 827–830. [Google Scholar] [CrossRef] [PubMed]
- European Parliament; Council of Europe: Strasbourg, France, 2008; 4.1.3. Buffer solutions. Ph. Eur. 2008, 6.0, 512.
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical modeling of release kinetics from supramolecular drug delivery systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Tudose, A.; Celia, C.; Di Marzio, L.; Cilurzo, F.; Mircioiu, C. Mathematical models as tools to predict the release kinetic of fluorescein from lyotropic colloidal liquid crystals. Materials 2019, 12, 693. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Sample | PVA:CS % | L-arg % | Caff % | LCL-arg ± SD % | LCCaff ± SD % | EEL-arg ± SD % | EECaff ± SD % |
|---|---|---|---|---|---|---|---|
| PVA_L-arg:CS | 90:10 | 2 | - | 1.98 ± 0.0002 | - | 99.05 ± 0.2 | - |
| 75:25 | 2 | - | 1.98 ± 0.0003 | - | 99.13 ± 0.5 | - | |
| 60:40 | 2 | - | 1.99± 0.0002 | - | 99.05 ± 0.7 | - | |
| PVA_Caff:CS | 90:10 | - | 2 | - | 1.97 ± 0.0003 | - | 98.84 ± 0.5 |
| 75:25 | - | 2 | - | 1.97 ± 0.0003 | - | 98.80 ± 0.6 | |
| 60:40 | - | 2 | - | 1.98 ± 0.0002 | - | 99.16 ± 0.3 | |
| PVA_L-arg_Caff:CS | 90:10 | 2 | 2 | - | 1.99 ± 0.0002 | - | 99.16 ± 0.3 |
| 75:25 | 2 | 2 | - | 1.97 ± 0.0002 | - | 98.89 ± 0.3 | |
| 60:40 | 2 | 2 | - | 1.99 ± 0.0001 | - | 99.27 ± 0.1 | |
| PVA_Z_L-arg:CS | 75:25 | 2 | - | 1.92 ± 0.001 | - | 95.88 ± 0.46 | - |
| PVA_Z_Caff:CS | 75:25 | - | 2 | - | 1.84 ± 0.003 | - | 91.80 ± 1.03 |
| PVA_Z_L-arg_Caff:CS | 75:25 | 2 | 2 | - | 1.70 ± 0.003 | - | 85.09 ± 1.02 |
| Kinetic Model | Model Coefficients * | Sample | |||
|---|---|---|---|---|---|
| PVA_L-arg:CS 90:10 | PVA_L-arg:CS 75:25 | PVA_L-arg:CS 60:40 | PVA_Z_L-arg:CS 75:25 | ||
| Zero-order | K0 (µg/h) | 34.6604 | 33.4181 | 33.0437 | 30.0594 |
| R2 | 0.7397 | 0.8173 | 0.7749 | 0.9400 | |
| AIC | 90.4906 | 86.1915 | 89.5444 | 68.7646 | |
| First-order | K1 (h−1) | 1.5081 | 1.1985 | 1.4667 | 0.7091 |
| R2 | 0.9540 | 0.9434 | 0.9098 | 0.9893 | |
| AIC | 52.0653 | 54.4257 | 59.4848 | 34.4222 | |
| Higuchi | KH (h−0.5) | 68.4502 | 58.6092 | 60.0338 | 50.3189 |
| R2 | 0.9207 | 0.9626 | 0.9395 | 0.9935 | |
| AIC | 67.5030 | 58.0864 | 66.1111 | 36.3008 | |
| Korsmeyer–Peppas | n | 0.31 | 0.35 | 0.30 | 0.54 |
| KP (h−n) | 71.1471 | 65.8352 | 70.2094 | 48.8744 | |
| R2 | 0.9728 | 0.9890 | 0.9898 | 0.9937 | |
| AIC | 47.2008 | 35.1684 | 33.1479 | 30.8432 | |
| Kinetic Model | Model Coefficients * | Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PVA_Caff:CS 90:10 | PVA_Caff:CS 75:25 | PVA_Caff:CS 60:40 | PVA_L-arg_Caff:CS 90:10 | PVA_L-arg_Caff:CS 75:25 | PVA_L-arg_Caff:CS 60:40 | PVA_Z_Caff:CS 75:25 | PVA_Z_L-arg_Caff:CS 75:25 | ||
| Zero-order | K0 (µg/h) | 31.7189 | 33.2027 | 32.3685 | 29.8304 | 30.4852 | 31.0108 | 29.0580 | 26.4436 |
| R2 | 0.8904 | 0.8328 | 0.8716 | 0.9540 | 0.9480 | 0.9445 | 0.9688 | 0.9769 | |
| AIC | 81.7390 | 86.3340 | 83.4886 | 69.4658 | 70.5143 | 68.7899 | 61.7122 | 49.9559 | |
| First-order | K1 (h−1) | 0.9182 | 1.1136 | 1.0039 | 0.6955 | 0.7318 | 0.7357 | 0.6162 | 0.4852 |
| R2 | 0.8788 | 0.8905 | 0.8873 | 0.9630 | 0.9658 | 0.9754 | 0.9732 | 0.9695 | |
| AIC | 62.8648 | 61.9319 | 62.1767 | 49.2599 | 48.8112 | 46.1660 | 46.1221 | 47.3676 | |
| Higuchi | KH (h−0.5) | 54.6274 | 58.0423 | 56.1032 | 49.4901 | 50.9705 | 51.2613 | 48.1464 | 43.0442 |
| R2 | 0.9803 | 0.9651 | 0.9772 | 0.9939 | 0.9924 | 0.9855 | 0.9824 | 0.9609 | |
| AIC | 51.2550 | 59.7086 | 54.0957 | 28.2244 | 30.6220 | 43.9314 | 47.4270 | 59.1188 | |
| Korsmeyer–Peppas | n | 0.38 | 0.33 | 0.36 | 0.54 | 0.55 | 0.58 | 0.64 | 0.78 |
| KP (h−n) | 60.0406 | 66.2640 | 62.3882 | 48.3429 | 48.9719 | 49.0189 | 42.6281 | 33.3417 | |
| R2 | 0.9878 | 0.9915 | 0.9903 | 0.9953 | 0.9933 | 0.9878 | 0.9925 | 0.9838 | |
| AIC | 35.0171 | 30.6338 | 32.2345 | 24.5002 | 29.6927 | 39.0642 | 31.5291 | 41.1629 | |
| Sample | PVA (wt%) | CS (wt%) | L-arg (wt%) | Caff (wt%) | L-arg–Caff (wt%) | Z (wt%) |
|---|---|---|---|---|---|---|
| PVA_L-arg:CS 90:10 | 90 | 10 | 2 | - | - | - |
| PVA_L-arg:CS 75:25 | 75 | 25 | 2 | - | - | - |
| PVA_L-arg:CS 60:40 | 60 | 40 | 2 | - | - | - |
| PVA_Caff:CS 90:10 | 90 | 10 | - | 2 | - | - |
| PVA_Caff:CS 75:25 | 75 | 25 | - | 2 | - | - |
| PVA_Caff:CS 60:40 | 60 | 40 | - | 2 | - | - |
| PVA_L-arg_Caff:CS 90:10 | 90 | 10 | - | - | 2 | - |
| PVA_L-arg_Caff:CS 75:25 | 75 | 25 | - | - | 2 | - |
| PVA_L-arg_Caff:CS 60:40 | 60 | 40 | - | - | 2 | - |
| PVA_Z_L-arg:CS 75:25 | 75 | 25 | 2 | - | - | 1 |
| PVA_Z_Caff:CS 75:25 | 75 | 25 | - | 2 | - | 1 |
| PVA_Z_L-arg_Caff:CS 75:25 | 75 | 25 | - | - | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherman, S.P.; Biliuță, G.; Bele, A.; Ipate, A.M.; Baron, R.I.; Ochiuz, L.; Șpac, A.F.; Zavastin, D.E. Biomaterials Based on Chitosan and Polyvinyl Alcohol as a Drug Delivery System with Wound-Healing Effects. Gels 2023, 9, 122. https://doi.org/10.3390/gels9020122
Gherman SP, Biliuță G, Bele A, Ipate AM, Baron RI, Ochiuz L, Șpac AF, Zavastin DE. Biomaterials Based on Chitosan and Polyvinyl Alcohol as a Drug Delivery System with Wound-Healing Effects. Gels. 2023; 9(2):122. https://doi.org/10.3390/gels9020122
Chicago/Turabian StyleGherman, Simona Petronela, Gabriela Biliuță, Adrian Bele, Alina Mirela Ipate, Raluca Ioana Baron, Lăcrămioara Ochiuz, Adrian Florin Șpac, and Daniela Elena Zavastin. 2023. "Biomaterials Based on Chitosan and Polyvinyl Alcohol as a Drug Delivery System with Wound-Healing Effects" Gels 9, no. 2: 122. https://doi.org/10.3390/gels9020122
APA StyleGherman, S. P., Biliuță, G., Bele, A., Ipate, A. M., Baron, R. I., Ochiuz, L., Șpac, A. F., & Zavastin, D. E. (2023). Biomaterials Based on Chitosan and Polyvinyl Alcohol as a Drug Delivery System with Wound-Healing Effects. Gels, 9(2), 122. https://doi.org/10.3390/gels9020122