Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections
Abstract
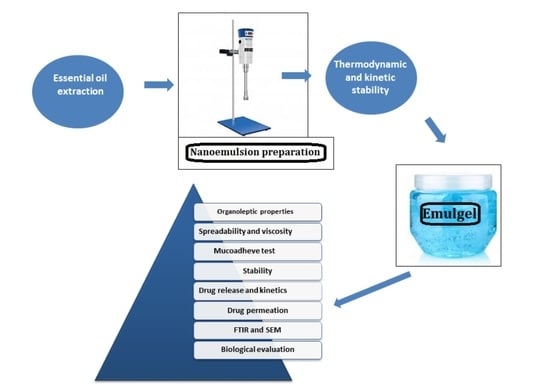
:1. Introduction
2. Results and Discussion
2.1. HLB Values of Different Smix Ratios
2.2. Optimization of Nanoemulsion
2.3. Globule Size, Polydispersity Index, and Zeta Potential
2.4. Kinetic Stability
2.5. Thermodynamic Stability
2.6. Optimization of Nanoemulgel
2.7. FTIR
2.8. Drug Content Analysis
2.9. Entrapment Efficiency of the Nanoemulsion and Nanoemulgel
2.10. Drug Release Profile of Nanoemulgel
2.11. Mechanism of Drug Release from the Essential Oil-Loaded Nanoemulgel
2.12. Buccal Mucosa Permeation of the Prepared Nanoemulgel
2.13. Mucoadhesive Test
2.14. Skin Irritation Test
2.15. Biological Evaluation of Nanoemulgel
2.15.1. Antimicrobial Activity
2.15.2. Antiquorum Sensing Activity
2.16. Scanning Electron Microscopy of the Essential-Loaded Nanoemulgel
2.17. Kinetic and Thermodynamic Stability of Nanoemulgel
3. Conclusions
4. Material and Methods
4.1. Chemicals and Bacterial Strains
4.2. Essential Oil Extraction
4.3. Determination of HLB values
4.4. Pseudo Ternary Phase Diagram
4.5. Preparation of Nanoemulsion
4.6. Globules Size
4.7. Stability of Nanoemulsion
4.7.1. Kinetic Stability
4.7.2. Thermodynamic Stability
4.8. Preparation of Nanoemulgel
4.8.1. Preparation of the Gel
4.8.2. Incorporation of the Prepared Gel into Already Prepared Nanoemulsion
4.9. Optimization of the Nanoemulgel Formulation
4.10. Characterization of Nanoemulgel
4.10.1. FTIR Analysis
4.10.2. Viscosity
4.10.3. Physical Appearance
4.10.4. pH Determination
4.10.5. Spreadability
4.10.6. Scanning Electron Microscopy of Nanoemulgel
4.10.7. Muco-Adhesion Test
4.10.8. Drug Contents Analysis
4.10.9. Encapsulation Efficiency of Nanoemulsion
4.10.10. Drug Release Profile
4.10.11. Ex vivo Permeation
4.10.12. Mucosal and Skin Irritation Test
4.10.13. Stability Studies
4.11. Biological Evaluation of Nanoemulgel
4.11.1. Antimicrobial Property
4.11.2. Antiquorum Sensing Activity
4.12. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122. [Google Scholar] [PubMed]
- Santosh, A.B.R.; Muddana, K.; Bakki, S.R. Fungal infections of oral cavity: Diagnosis, management, and association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Irani, S. Orofacial bacterial infectious diseases: An update. J. Int. Soc. Prev. Community Dent. 2017, 7 (Suppl. S2), S61. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.H.N.; Samaranayake, L.P. Viral, bacterial, and fungal infections of the oral mucosa: Types, incidence, predisposing factors, diagnostic algorithms, and management. Periodontology 2000 2019, 80, 148–176. [Google Scholar] [CrossRef] [PubMed]
- Korona-Glowniak, I.; Skawinska-Bednarczyk, A.; Wrobel, R.; Pietrak, J.; Tkacz-Ciebiera, I.; Maslanko-Switala, M.; Krawczyk, D.; Bakiera, A.; Borek, A.; Malm, A.; et al. Streptococcus sobrinus as a Predominant Oral Bacteria Related to the Occurrence of Dental Caries in Polish Children at 12 Years Old. Int. J. Environ. Res. Public Health 2022, 19, 15005. [Google Scholar] [CrossRef]
- SriChinthu, K.K.; Pavithra, V.; Kumar, G.; Prasad, H.; Prema, P.; Yoithapprabhunath, T.R.; Rangarajan, N. Evaluation of gingival and periodontal status in obese and non-obese type II diabetic patients—A cross sectional study. Med. Pharm. Rep. 2021, 94, 94. [Google Scholar] [CrossRef] [PubMed]
- Chmit, M.; Kanaan, H.; Habib, J.; Abbass, M.; Mcheik, A.; Chokr, A. Antibacterial and antibiofilm activities of polysaccharides, essential oil, and fatty oil extracted from Laurus nobilis growing in Lebanon. Asian Pac. J. Trop. Med. 2014, 7, S546–S552. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Aue, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Singh, I.; Kaur, P.; Kaushal, U.; Kaur, V.; Shekhar, N. Essential Oils in Treatment and Management of Dental Diseases. Biointerf. Res. Appl. Chem. 2022, 12, 7267–7286. [Google Scholar]
- Dagli, N.; Dagli, R.; Mahmoud, R.S.; Baroudi, K. Essential oils, their therapeutic properties, and implication in dentistry: A review. J. Int. Soc. Prev. Community Dent. 2015, 5, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nabavi, S.M.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumari, R.; Mishra, S. Pharmacological properties and their medicinal uses of Cinnamomum: A review. J. Pharm. Pharmacol. 2019, 71, 1735–1761. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.J.; Lee, S.G.; Lee, H.S. Acaricidal and insecticidal activities of essential oils of Cinnamomum zeylanicum barks cultivated from France and India against Dermatophagoides spp., Tyrophagus putrescentiae and Ricania sp. Appl. Biol. Chem. 2017, 60, 259–264. [Google Scholar] [CrossRef]
- Goel, N.; Rohilla, H.; Singh, G.; Punia, P. Antifungal activity of cinnamon oil and olive oil against Candida Spp. isolated from blood stream infections. J. Clin. Diagn. Res. 2016, 10, DC09. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid.-Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Mann, B.; Singh, R.; Athira, S.; Kumar, R.; Sharma, R. Chemistry and functionality of clove oil nanoemulsions. In Clove (Syzygium Aromaticum); Academic Press: Cambridge, MA, USA, 2022; pp. 81–101. [Google Scholar]
- Yoo, J.H.; Baek, K.H.; Heo, Y.S.; Yong, H.I.; Jo, C. Synergistic bactericidal effect of clove oil and encapsulated atmospheric pressure plasma against Escherichia coli O157: H7 and Staphylococcus aureus and its mechanism of action. Food Microbiol. 2021, 93, 103611. [Google Scholar] [CrossRef]
- Barradas, T.N.; de Holanda e Silva, K.G. Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 2021, 19, 1153–1171. [Google Scholar] [CrossRef]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut oil nanoemulsion loaded with a statin hypolipidemic drug for management of burns: Formulation and in vivo evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A promising phase in drug delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Aslani, A.; Zolfaghari, B.; Davoodvandi, F. Design, formulation and evaluation of an oral gel from Punica granatum flower extract for the treatment of recurrent aphthous stomatitis. Adv. Pharm. Bull. 2016, 6, 391. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Sindi, A.M.; Bakhaidar, R.B.; Zaki, R.M.; Abualsunun, W.A.; Alkhalidi, H.M.; Bahmdan, R.H.; Md, S.; Hassan, A.H. Formulation and optimization of neomycin Sulfate—Thioctic acid loaded in a eucalyptus oil self-nanoemulsion to enhance the beneficial activity of the substances and limit the side effects associated with the treatment of hepatic coma. J. Drug Deliv. Sci. Technol. 2021, 61, 102108. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent update on nanoemulgel as topical drug delivery system. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The multifaceted role of curcumin in advanced nanocurcumin form in the treatment and management of chronic disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef] [PubMed]
- Aithal, G.C.; Nayak, U.Y.; Mehta, C.; Narayan, R.; Gopalkrishna, P.; Pandiyan, S.; Garg, S. Localized In Situ Nanoemulgel Drug Delivery System of Quercetin for Periodontitis: Development and Computational Simulations. Molecules 2018, 23, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, V.; Saraf, S.A. Rheological studies on solid lipid nanoparticle based carbopol gels of aceclofenac. Colloids Surf. B Biointerfaces 2012, 92, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Nayak, A.K.; Nanda, U. Topical gels of lidocaine HCl using cashew gum and Carbopol 940: Preparation and in vitro skin permeation. Int. J. Biol. Macromol. 2013, 62, 514–517. [Google Scholar] [CrossRef]
- Ullah, N.; Amin, A.; Alamoudi, R.A.; Rasheed, S.A.; Alamoudi, R.A.; Nawaz, A.; Raza, M.; Nawaz, T.; Ishtiaq, S.; Abbas, S.S. Fabrication and Optimization of Essential-Oil-Loaded Nanoemulsion Using Box–Behnken Design against Staphylococos aureus and Staphylococos epidermidis Isolated from Oral Cavity. Pharmaceutics 2022, 14, 1640. [Google Scholar] [CrossRef]
- Halnor, V.V.; Pande, V.V.; Borawake, D.D.; Nagare, H.S. Nanoemulsion: A novel platform for drug delivery system. J. Mat. Sci. Nanotechol. 2018, 6, 104. [Google Scholar]
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A promising novel formulation for treatment of skin ailments. Polym. Bull. 2022, 79, 4441–4465. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, characterization and evaluation of anti-inflammatory and anti-nociceptive effects of brucine-loaded nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Badawi, N.M.; Attia, D.A.; El-Nabarawi, M.A.; Elmazar, M.M.; Mousa, S.A. Efficacy of pomegranate extract loaded solid lipid nanoparticles transdermal emulgel against Ehrlich ascites carcinoma. Nanomed. Nanotechnol. Biol. Med. 2022, 39, 102466. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, T.; Iqbal, M.; Khan, B.A.; Nawaz, A.; Hussain, T.; Hosny, K.M.; Abualsunun, W.A.; Rizg, W.Y. Development and Optimization of Acriflavine-Loaded Polycaprolactone Nanoparticles Using Box–Behnken Design for Burn Wound Healing Applications. Polymers 2021, 14, 101. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, F.J.; Bedi, S.; Sharma, S.; Umar, S.; Ansari, M.A. A novel nanoformulation development of eugenol and their treatment in inflammation and periodontitis. Saudi Pharm. J. 2019, 27, 778–790. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Z.; Geng, Y. Anti-allergic effect of Artemisia extract in rats. Exp. Ther. Med. 2016, 12, 1130–1134. [Google Scholar] [CrossRef] [Green Version]
- Islam, N.; Irfan, M.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.K.; Khan, I.U.; Rasul, A.; Khan, S.U.; Alqahtani, A.M.; Ikram, M.; et al. Improved bioavailability of ebastine through development of transfersomal oral films. Pharmaceutics 2021, 13, 1315. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, J.; Lu, B.; Lin, H.; Sarkar, R.; Wu, T.; Li, X. Nanoemulgel for improved topical delivery of desonide: Formulation design and characterization. AAPS PharmSciTech 2021, 22, 163. [Google Scholar] [CrossRef]
- Bayer, I.S. Recent advances in mucoadhesive interface materials, mucoadhesion characterization, and technologies. Advanc. Mat. Int. 2022, 9(18), 2200211. [Google Scholar] [CrossRef]
- Dave, R.S.; Goostrey, T.C.; Ziolkowska, M.; Czerny-Holownia, S.; Hoare, T.; Sheardown, H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J. Control Release. 2021, 336, 71–88. [Google Scholar] [CrossRef]
- Khan, M.A.; Pandit, J.; Sultana, Y.; Sultana, S.; Ali, A.; Aqil, M.; Chauhan, M. Novel carbopol-based transfersomal gel of 5-fluorouracil for skin cancer treatment: In vitro characterization and in vivo study. Drug Deliv. 2015, 22, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Ginting, E.V.; Retnaningrum, E.; Widiasih, D.A. Antibacterial activity of clove (Syzygium aromaticum) and cinnamon (Cinnamomum burmannii) essential oil against extended-spectrum β-lactamase-producing bacteria. Vet. World 2021, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Zhang, T.; Yuan, Y.; Lin, S.; Xu, J.; Ye, H. Effects of cinnamaldehyde on Escherichia coli and Staphylococcus aureus membrane. Food Control. 2014, 47, 196–202. [Google Scholar] [CrossRef]
- Simões, A.; Miranda, M.; Cardoso, C.; Veiga, F.; Vitorino, C. Rheology by design: A regulatory tutorial for analytical method validation. Pharmaceutics 2020, 12, 820. [Google Scholar] [CrossRef]
- Dano, M.E.; dos Santos, R.S.; da Silva, J.B.; Junqueira, M.V.; de Souza Ferreira, S.B.; Bruschi, M.L. Design of emulgel platforms for local propolis delivery: The influence of type and concentration of carbomer. J. Mol. Liq. 2021, 334, 116025. [Google Scholar] [CrossRef]
- Gadhave, A. Determination of hydrophilic-lipophilic balance value. Int. J. Sci. Res. 2014, 3, 573–575. [Google Scholar]
- Akhtar, J.; Siddiqui, H.H.; Fareed, S.; Badruddeen Khalid, M.; Aqil, M. Nanoemulsion: For improved oral delivery of repaglinide. Drug Deliv. 2016, 23, 2026–2034. [Google Scholar] [CrossRef]
- Chong, W.T.; Tan, C.P.; Cheah, Y.K.; BLajis, A.F.; Habi Mat Dian, N.L.; Kanagaratnam, S.; Lai, O.M. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS ONE 2018, 13, e0202771. [Google Scholar] [CrossRef] [Green Version]
- Alkhanjaf, A.A.M.; Athar, M.T.; Ullah, Z.; Umar, A.; Shaikh, I.A. In Vitro and In Vivo Evaluation of a Nano-Tool Appended Oilmix (Clove and Tea Tree Oil) Thermosensitive Gel for Vaginal Candidiasis. J. Funct. Biomater. 2022, 13, 203. [Google Scholar] [CrossRef]
- Tasneem, R.; Khan, H.M.; Zaka, H.S.; Khan, P. Development and cosmeceutical evaluation of topical emulgel containing Albizia lebbeck bark extract. J. Cosmet. Dermatol. 2022, 21, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kanoujia, J.; Parashar, P.; Arya, M.; Tripathi, C.B.; Sinha, V.R.; Saraf, S.K.; Saraf, S.A. Assessment of improved buccal permeation and bioavailability of felodipine microemulsion-based cross-linked polycarbophil gel. Drug Deliv. Transl. Res. 2018, 8, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Sabri, H.S.; Alia, W.K.; Abdullahb, B.H.; Al-Anic, W.M. Formulation design and evaluation of anti-microbial activity of emulgel containing essential oil of Myrtus communis L. Int. J. Pharm. Sci. Rev. Res. 2016, 40, 271–277. [Google Scholar]
- Malyadri, T. Formulation development and evaluation of Luliconazole Topical Emulgel. Int. J. Indig. Herbs Drugs 2021, 6, 79–87. [Google Scholar]
- Pagano, C.; Baiocchi, C.; Beccari, T.; Blasi, F.; Cossignani, L.; Ceccarini, M.R.; Orabona, C.; Orecchini, E.; Di Raimo, E.; Primavilla, S.; et al. Emulgel loaded with flaxseed extracts as new therapeutic approach in wound treatment. Pharmaceutics 2021, 13, 1107. [Google Scholar] [CrossRef]
- Permana, A.D.; Utami, R.N.; Layadi, P.; Himawan, A.; Juniarti, N.; Anjani, Q.K.; Utomo, E.; Mardikasari, S.A.; Arjuna, A.; Donnelly, R.F. Thermosensitive and mucoadhesive in situ ocular gel for effective local delivery and antifungal activity of itraconazole nanocrystal in the treatment of fungal keratitis. Int. J. Pharm. 2021, 602, 120623. [Google Scholar] [CrossRef]
- Javed, H.; Shah, S.N.; Iqbal, F.M. Formulation development and evaluation of diphenhydramine nasal nano-emulgel. AAPS pharmscitech 2018, 19, 1730–1743. [Google Scholar] [CrossRef]
- da Silva Campelo, M.; Melo, E.O.; Arrais, S.P.; do Nascimento, F.B.; Gramosa, N.V.; de Aguiar Soares, S.; Ribeiro, M.E.; da Silva, C.R.; Júnior, H.V.; Ricardo, N.M. Clove essential oil encapsulated on nanocarrier based on polysaccharide: A strategy for the treatment of vaginal candidiasis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125732. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.H.; Lee, J. Direct one-pot synthesis of cinnamaldehyde immobilized on gold nanoparticles and their antibiofilm properties. Colloids Surf. B Biointerfaces 2017, 160, 639–648. [Google Scholar] [CrossRef]
- Dario, M.F.; Oliveira, C.A.; Cordeiro, L.R.; Rosado, C.; Inês de Fátima, A.M.; Maçôas, E.; Santos, M.S.; da Piedade, M.E.; Baby, A.R.; Velasco, M.V. Stability and safety of quercetin-loaded cationic nanoemulsion: In vitro and in vivo assessments. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 591–599. [Google Scholar] [CrossRef]
- Torregrosa, A.; Ochoa-Andrade, A.T.; Parente, M.E.; Vidarte, A.; Guarinoni, G.; Savio, E. Development of an emulgel for the treatment of rosacea using quality by design approach. Drug Dev. Ind. Pharm. 2020, 46, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Ammanage, A.; Rodriques, P.; Kempwade, A.; Hiremath, R. Formulation and evaluation of buccal films of piroxicam co-crystals. Future J. Pharm. Sci. 2020, 6, 16. [Google Scholar] [CrossRef]
- Liu, S.; Jin, M.N.; Quan, Y.S.; Kamiyama, F.; Kusamori, K.; Katsumi, H.; Sakane, T.; Yamamoto, A. Transdermal delivery of relatively high molecular weight drugs using novel self-dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. Eur. J. Pharm. Biopharm. 2014, 86, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Ahad, A.; Al-Saleh, A.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I.; Raish, M.; Yassin, A.E.; Alam, M.A. Pharmacodynamic study of eprosartan mesylate-loaded transfersomes Carbopol® gel under Dermaroller® on rats with methyl prednisolone acetate-induced hypertension. Biomed. Pharmacother. 2017, 89, 177–184. [Google Scholar] [CrossRef]
- Sohail, M.; Naveed, A.; Abdul, R.; Khan, H.M.; Khan, H. An approach to enhanced stability: Formulation and characterization of Solanum lycopersicum derived lycopene based topical emulgel. Saudi Pharm. J. 2018, 26, 1170–1177. [Google Scholar] [CrossRef]
- Amin, A.; Hanif, M.; Abbas, K.; Ramzan, M.; Rasheed, A.; Zaman, A.; Pieters, L. Studies on effects of umbelliferon derivatives against periodontal bacteria; antibiofilm, inhibition of quorum sensing and molecular docking analysis. Microb. Pathog. 2020, 144, 104184. [Google Scholar] [CrossRef]
- Rafey, A.; Amin, A.; Kamran, M.; Haroon, U.; Farooq, K.; Foubert, K.; Pieters, L. Plant Origin Antibiotics Against Periodontal Infections; Antibiofilm, Anti-Quorum Sensing, Molecular Docking Studies and Characterization of Active Constituents. Antibiotics 2021, 10, 1504. [Google Scholar] [CrossRef]







| Smix Ratio | HLB Values |
|---|---|
| 1:1 | 9.65 |
| 1:2 | 7.83 |
| 1:3 | 6.975 |
| 1:4 | 6.44 |
| 2:1 | 11.76 |
| 3:1 | 12.33 |
| 4:1 | 12.86 |
| F/Code | Composition (% w/w) | P.S (nm) | Z.P (mV) | PDI |
|---|---|---|---|---|
| N1 | Oil = 10% Smix = 2.5% w/w water = 87.5% w/w | 152 ± 3 | −19.54 ± 2 | 0.309 ± 0.023 |
| N2 | Oil = 15% w/w Smix = 3% w/w Water = 82% w/w | 227 ± 2 | −17 ± 3 | 0.267 ± 0.032 |
| N3 | Oil = 20% w/w Smix = 3.5% w/w Water = 76.5 | 257.6 ± 4 | −14 ± 2 | 0.279 ± 0.055 |
| N4 | Oil = 25% w/w Smix = 3.5% w/w Water = 71.5% w/w | 325 ± 3 | −12.75 ± 2 | 0.076 ± 0.098 |
| Parameters | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Color | White | White | White | White |
| Consistency | Good | Good | Good | Good |
| Homogeneity | Excellent | Excellent | Excellent | Excellent |
| pH | 6.8 ± 1 | 6.8 ± 0.5 | 6.8 ± 0.5 | 6.8 ± 0.5 |
| Viscosity (mPa·S) | 62,035 ± 10 | 65,311 ± 7 | 91,306 ± 15 | 96,432 ± 10 |
| Spreadability (g·cm/s) | 38 ± 1 | 36 ± 0.5 | 31 ± 2 | 27 ± 2 |
| Formulation | Active | Zero Order | First Order | Higuchi Model | Korsmeyer–Peppas Model | N | Best Fit Model |
|---|---|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | ||||
| Nanoemulgel | Cinn | 0.9539 | 0.9641 | 0.9709 | 0.9904 | 0.507 | Korsmeyer–Peppas models |
| Eug | 0.9515 | 0.9719 | 0.9701 | 0.9905 | 0.522 |
| Formulation | Erythema | Edema | ||||
|---|---|---|---|---|---|---|
| 0 h | 24 h | 48 h | 0 h | 24 h | 48 h | |
| Positive control | 0.00 | 3.7 | 2.80 | 0.00 | 1.69 | 2.13 |
| NEG | 0.00 | 0.30 | 0.00 | 0.00 | 0.00 | 0.00 |
| Negative control | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| F/Code | Concentration (%) | Strain | Zone of Inhibition (mm) |
|---|---|---|---|
| 1 ENEG | 3 | S. epidermidis | 19 ± 1 |
| ENEG | 3 | S. aureus | 19 ± 0.5 |
| ENEG | 1.5 | S. epidermidis | 6 ± 1 |
| ENEG | 1.5 | S. aureus | 6 ± 1 |
| ENEG | 3 | Pseudomonas aeruginosa | 4 ± 1 |
| ENEG | 3 | Bacillus chungangensis | 0 |
| ENEG | 3 | Bacillus paramycoides | 0 |
| ENEG | 3 | Bacillus chungangensis | 2 ± 1 |
| ENEG | 3 | Paenibacillus dendritiformis | 0 |
| ENEG | 3 | Candida albicans | 6 ± 1 |
| Formulation | Strain | Zone of Inhibition (mm) |
|---|---|---|
| 1 ENEG | 3 CV | 20 ± 1 |
| 2 NEG | CV | 0 |
| Weak | Homogeneity | pH | Spreadability | Viscosity (mPa·S) | Centrifugation | Color |
|---|---|---|---|---|---|---|
| 0 | Homogeneous | 6.8 ± 0.1 | 37 ± 0.5 | 65311 ± 5 | Stable | White |
| 3rd | Homogeneous | 6.8 ± 0.1 | 37 ± 0.3 | 65612 ± 8 | Stable | White |
| 6th | Homogeneous | 6.8 ± 0.1 | 37 ± 0.5 | 65730 ± 10 | Stable | White |
| 9th | Homogeneous | 6.8 ± 0.1 | 37 ± 0.1 | 65921 ± 5 | Stable | White |
| 12th | Homogeneous | 6.8 ± 0.1 | 37 ± 0.1 | 65992 ± 8 | Stable | White |
| Weak | Homogeneity | pH | Spreadability | Viscosity (mPa·S) | Centrifugation | Color |
|---|---|---|---|---|---|---|
| 0 | Homogeneous | 6.8 ± 0.1 | 37 ± 0.5 | 65,311 ± 10 | Stable | White |
| 3rd | Homogeneous | 6.8 ± 0.1 | 37 ± 0.1 | 66,943 ± 6 | Stable | White |
| 6th | Homogeneous | 6.8 ± 0.1 | 36 ± 0.2 | 69,211 ± 7 | Stable | White |
| 9th | Homogeneous | 6.8 ± 0.1 | 33 ± 0.3 | 71,388 ± 9 | Stable | White |
| 12th | Homogeneous | 6.8 ± 0.1 | 30 ± 0.5 | 75,218 ± 5 | Stable | White |
| Weak | Homogeneity | pH | Spreadability | Viscosity (mPa·S) | Centrifugation | Color |
|---|---|---|---|---|---|---|
| 0 | Homogeneous | 6.8 ± 0.1 | 37 ± 0.5 | 65,311 ± 11 | Stable | White |
| 3rd | Homogeneous | 6.8 ± 0.1 | 37 ± 0.8 | 62,230 ± 8 | Stable | Off white |
| 6th | Heterogeneous | 6.8 ± 0.1 | 38 ± 1 | 58,963 ± 9 | Unstable | Dark brown |
| 9th | Heterogeneous | 6.8 ± 0.1 | 40 ± 0.5 | 55,871 ± 5 | Unstable | Dark brown |
| 12th | Heterogeneous | 6.8 ± 0.1 | 41 ± 0.9 | 48,222 ± 9 | Unstable | Dark brown |
| HLB | Application |
|---|---|
| 4–6 | w/o emulsifier |
| 7–9 | Wetting agents |
| 8–18 | o/w emulsifier |
| 13–15 | Detergent |
| 10–18 | Solubilizes |
| Ingredients (% w/w) | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Essential oil (% w/w) | 1.5 | 1.5 | 1.5 | 1.5 |
| Carbopol 940 (% w/w) | 0.5 | 1 | 1.5 | 2 |
| Methyl paraben | 0.01 | 0.01 | 0.01 | 0.01 |
| Propyl paraben | 0.05 | 0.05 | 0.05 | 0.05 |
| Triethanolamine | q.s. | q.s. | q.s. | q.s. |
| Distilled water | q.s. | q.s. | q.s. | q.s. |
| Evaluation of Dermal Reaction | |||
|---|---|---|---|
| Value | Erythema | Value | Edema Formation |
| 0 | No erythema | 0 | No edema |
| 1 | Very slight erythema | 1 | Very slight edema |
| 2 | Slight erythema | 2 | Slight edema |
| 3 | Moderate to severe erythema | 3 | Moderate to severe edema |
| 4 | Severe erythema | 4 | Severe edema |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, N.; Amin, A.; Farid, A.; Selim, S.; Rashid, S.A.; Aziz, M.I.; Kamran, S.H.; Khan, M.A.; Rahim Khan, N.; Mashal, S.; et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels 2023, 9, 252. https://doi.org/10.3390/gels9030252
Ullah N, Amin A, Farid A, Selim S, Rashid SA, Aziz MI, Kamran SH, Khan MA, Rahim Khan N, Mashal S, et al. Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels. 2023; 9(3):252. https://doi.org/10.3390/gels9030252
Chicago/Turabian StyleUllah, Niamat, Adnan Amin, Arshad Farid, Samy Selim, Sheikh Abdur Rashid, Muhammad Imran Aziz, Sairah Hafeez Kamran, Muzammil Ahmad Khan, Nauman Rahim Khan, Saima Mashal, and et al. 2023. "Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections" Gels 9, no. 3: 252. https://doi.org/10.3390/gels9030252
APA StyleUllah, N., Amin, A., Farid, A., Selim, S., Rashid, S. A., Aziz, M. I., Kamran, S. H., Khan, M. A., Rahim Khan, N., Mashal, S., & Mohtasheemul Hasan, M. (2023). Development and Evaluation of Essential Oil-Based Nanoemulgel Formulation for the Treatment of Oral Bacterial Infections. Gels, 9(3), 252. https://doi.org/10.3390/gels9030252