An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication and Swelling-Shrinking Properties of PZ-PAA@Fe3+ Bi-Layer Hydrogel
2.2. pH and Thermal Induced Sequential Two-Stage Bending of PZ-PAA@Fe3+ Bi-Layer Hydrogel
2.3. Mechanical Properties of the Bi-Layer Hydrogel
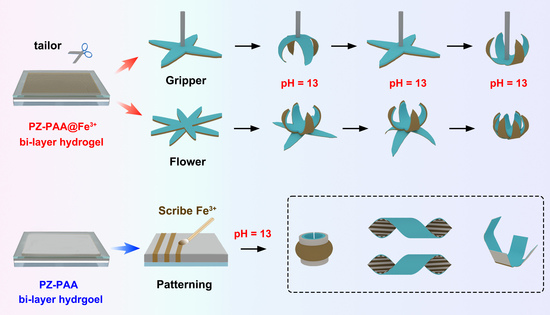
2.4. Programmable Shape Transformations by Locally Fe3+ Coordinating Patter and Biomimetic Applications
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Bi-Layer Hydrogel Preparation
4.3. Patterned Heterogeneous Hydrogel Preparation
4.4. Characterization of Hydrogels
4.5. Swelling Behaviors of Hydrogels
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, A.; Manna, K.; Pal, S. Recent advances in various stimuli-responsive hydrogels: From synthetic designs to emerging healthcare applications. Mater. Chem. Front. 2022, 6, 2338–2385. [Google Scholar] [CrossRef]
- Liu, X.Y.; Liu, J.; Lin, S.T.; Zhao, X.H. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Deng, Z.X.; Yu, R.; Guo, B. Stimuli-responsive conductive hydrogels: Design, properties, and applications. Mater. Chem. Front. 2021, 5, 2092–2123. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, Y.; Jin, S.; He, Z.; Duan, G.; Gu, H.; Xu, H.; Cao, X.; Ma, C.; Wu, J. Regenerable bacterial killing–releasing ultrathin smart hydrogel surfaces modified with zwitterionic polymer brushes. e-Polymers 2022, 22, 719–732. [Google Scholar] [CrossRef]
- An, B.; Wang, Y.; Huang, Y.; Wang, X.; Liu, Y.; Xun, D.; Church, G.M.; Dai, Z.; Yi, X.; Tang, T.C.; et al. Engineered living materials for sustainability. Chem. Rev. 2023, 123, 2349–2419. [Google Scholar] [CrossRef]
- Ouyang, Y.; Huang, G.; Cui, J.; Zhu, H.; Yan, G.; Mei, Y. Advances and challenges of hydrogel materials for robotic and sensing applications. Chem. Mater. 2022, 34, 9307–9328. [Google Scholar] [CrossRef]
- He, H.; Li, H.; Pu, A.; Li, W.; Ban, K.; Xu, L. Hybrid assembly of polymeric nanofiber network for robust and electronically conductive hydrogels. Nat. Commun. 2023, 14, 759. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, L.; Fu, H. Research progress on constructing self-supporting cathodes of synergistic electrocatalytic ORR/OER for zinc-air batteries. J. Mater. Chem. A 2023, 11, 4400–4427. [Google Scholar] [CrossRef]
- Amit, S.; Mansoor, M.A. Stimuli-Responsive Drug Delivery Systems; Royal Society of Chemistry: London, UK, 2018. [Google Scholar] [CrossRef]
- Li, Q.; Wen, C.; Yang, J.; Zhou, X.; Zhu, Y.; Zheng, J.; Chen, G.; Bai, J.; Xu, T.; Ji, J.; et al. Zwitterionic biomaterials. Chem. Rev. 2022, 122, 17073–17154. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, J.; Zhang, Y.; Wang, T.; Sun, W.; Tong, Z. Programmable and bidirectional bending of soft actuators based on Janus structure with sticky tough PAA-clay hydrogel. ACS Appl. Mater. Interfaces 2017, 9, 11866–11873. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, Y.; Zhong, M.; Chen, H.; Zhang, Y.; Yang, J.; Zheng, J. Salt-responsive bilayer hydrogels with pseudo-double-network structure actuated by polyelectrolyte and antipolyelectrolyte effects. ACS Appl. Mater. Interfaces 2017, 9, 20843–20851. [Google Scholar] [CrossRef]
- Xiao, S.; He, X.; Zhao, Z.; Huang, G.; Yan, Z.; He, Z.; Zhao, Z.; Chen, F.; Yang, J. Strong anti-polyelectrolyte zwitterionic hydrogels with superior self-recovery, tunable surface friction, conductivity, and antifreezing properties. Eur. Polym. J. 2021, 148, 110350. [Google Scholar] [CrossRef]
- Xiao, S.; He, X.; Qian, J.; Wu, X.; Huang, G.; Jiang, H.; He, Z.; Yang, J. Natural lipid inspired hydrogel-organogel bilayer actuator with a tough interface and multiresponsive, rapid, and reversible behaviors. Ind. Eng. Chem. Res. 2020, 59, 7646–7658. [Google Scholar] [CrossRef]
- Li, J.; Ma, Q.; Xu, Y.; Yang, M.; Wu, Q.; Wang, F.; Sun, P. Highly bidirectional bendable actuator engineered by LCST-UCST bilayer hydrogel with enhanced interface. ACS Appl. Mater. Interfaces 2020, 12, 55290–55298. [Google Scholar] [CrossRef]
- Wei, X.; Chen, L.; Wang, Y.; Sun, Y.; Ma, C.; Yang, X.; Jiang, S.; Duan, G. An electrospinning anisotropic hydrogel with remotely-controlled photo-responsive deformation and long-range navigation for synergist actuation. Chem. Eng. J. 2022, 433, 134258. [Google Scholar] [CrossRef]
- Shin, Y.; Choi, M.Y.; Choi, J.; Na, J.H.; Kim, S.Y. Design of an electro-stimulated hydrogel actuator system with fast flexible folding deformation under a low electric field. ACS Appl. Mater. Interfaces 2021, 13, 15633–15646. [Google Scholar] [CrossRef]
- Tang, J.; Yin, Q.; Qiao, Y.; Wang, T. Shape morphing of hydrogels in alternating magnetic field. ACS Appl. Mater. Interfaces 2019, 11, 21194–21200. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, H.; Eklund, A.; Guo, H.; Priimagi, A.; Ikkala, O. Feedback-controlled hydrogels with homeostatic oscillations and dissipative signal transduction. Nat. Nanotechnol. 2022, 17, 1303–1310. [Google Scholar] [CrossRef]
- Peng, Z.; Huang, J.; Guo, Z. Anisotropic Janus materials: From micro-/nanostructures to applications. Nanoscale 2021, 13, 18839–18864. [Google Scholar] [CrossRef]
- Noblin, X.; Rojas, N.O.; Westbrook, J.; Llorens, C.; Argentina, M.; Dumais, J. The fern sporangium: A unique catapult. Science 2012, 335, 1322. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Wang, S.; Zhou, J.; Zhang, D.; Xue, Y.; Yang, X.; Che, L.; Li, D.; Xiao, S.; Liu, S.; et al. Versatile and Simple Strategy for Preparing Bilayer Hydrogels with Janus Characteristics. ACS Appl. Mater. Interface 2022, 14, 4579–4587. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, Y.; Wu, J.; Wang, Y.; Chen, F.; Fan, P.; Zhong, M.; Xiao, S.; Zhang, D.; Yang, J.; et al. Dual-stimulus bilayer hydrogel actuators with rapid, reversible, bidirectional bending behaviors. J. Mater. Chem. C 2019, 7, 4970–4980. [Google Scholar] [CrossRef]
- Fusi, G.; Del Giudice, D.; Skarsetz, O.; Di Stefano, S.; Walther, A. Autonomous soft Robots empowered by chemical reaction networks. Adv. Mater. 2022, 35, 2209870. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Chen, Z.; Fang, S.; Zhu, Y.; Baughman, R.H.; Jiang, L. Tunable, fast, robust hydrogel actuators based on evaporation-programmed heterogeneous structures. Chem. Mater. 2017, 29, 9793–9801. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, Q.; Jeng, U.S.; Hsu, S.H. A biomimetic bilayer hydrogel actuator based on thermoresponsive gelatin methacryloyl–poly(N-isopropylacrylamide) hydrogel with three-dimensional printability. ACS Appl. Mater. Interfaces 2023, 15, 5798–5810. [Google Scholar] [CrossRef]
- Liu, W.; Geng, L.; Wu, J.; Huang, A.; Peng, X. Highly strong and sensitive bilayer hydrogel actuators enhanced by cross-oriented nanocellulose networks. Compos. Sci. Technol. 2022, 225, 109494. [Google Scholar] [CrossRef]
- Wei, X.; Xue, Y.; Sun, Y.; Chen, L.; Zhang, C.; Wu, Q.; Peng, S.; Ma, C.; Liu, Z.; Jiang, S.; et al. A robust anisotropic light-responsive hydrogel for ultrafast and complex biomimetic actuation via poly(pyrrole)-coated electrospun nanofiber. Chem. Eng. J. 2023, 452, 139373. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, M.; He, X.; Huang, L.; Zhang, Y.; Ren, B.; Zhong, M.; Chang, Y.; Yang, J.; Zheng, J. Dual salt-and thermoresponsive programmable bilayer hydrogel actuators with pseudo-interpenetrating double-network structures. ACS Appl. Mater. Interface 2018, 10, 21642–21653. [Google Scholar] [CrossRef]
- He, X.; Zhang, D.; Wu, J.; Wang, Y.; Chen, F.; Fan, P.; Zhong, M.; Xiao, S.; Yang, J. One-pot and one-step fabrication of salt-responsive bilayer hydrogels with 2D and 3D shape transformations. ACS Appl. Mater. Interface 2019, 11, 25417–25426. [Google Scholar] [CrossRef]
- Cui, H.; Pan, N.; Fan, W.; Liu, C.; Li, Y.; Xia, Y.; Sui, K. Ultrafast fabrication of gradient nanoporous all-polysaccharide films as strong, superfast, and multiresponsive actuators. Adv. Funct. Mater. 2019, 29, 1807692. [Google Scholar] [CrossRef]
- Liu, J.; Xu, W.; Kuang, Z.; Dong, P.; Yao, Y.; Wu, H.; Liu, A.; Ye, F. Gradient porous PNIPAM-based hydrogel actuators with rapid response and flexibly controllable deformation. J. Mater. Chem. C 2020, 8, 12092–12099. [Google Scholar] [CrossRef]
- Gregory, K.P.; Elliott, G.R.; Robertson, H.; Kumar, A.; Wanless, E.J.; Webber, G.B.; Craig, V.S.J.; Andersson, G.G.; Page, A.J. Understanding specific ion effects and the Hofmeister series. Phys. Chem. Chem. Phys. 2022, 24, 12682–12718. [Google Scholar] [CrossRef]
- Wang, Z.J.; Hong, W.; Wu, Z.L.; Zheng, Q. Site-specific pre-swelling-directed morphing structures of patterned hydrogels. Angew. Chem. 2017, 129, 16190–16194. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Xiao, S.; Shen, M.; Chen, F.; Fan, P.; Zhong, M.; Zheng, J. Salt-responsive zwitterionic polymer brushes with tunable friction and antifouling properties. Langmuir 2015, 31, 9125–9133. [Google Scholar] [CrossRef]
- Chen, H.; Yang, J.; Xiao, S.; Hu, R.; Bhaway, S.M.; Vogt, B.D.; Zhang, M.; Chen, Q.; Ma, J.; Chang, Y.; et al. Salt-responsive polyzwitterionic materials for surface regeneration between switchable fouling and antifouling properties. Acta Biomater. 2016, 40, 62–69. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Cao, X.; Zhao, Y.; Li, H.; Xiao, S.; Chen, Z.; Huang, G.; Sun, Y.; Liu, Z.; He, Z. An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus. Gels 2023, 9, 279. https://doi.org/10.3390/gels9040279
Zhang Y, Cao X, Zhao Y, Li H, Xiao S, Chen Z, Huang G, Sun Y, Liu Z, He Z. An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus. Gels. 2023; 9(4):279. https://doi.org/10.3390/gels9040279
Chicago/Turabian StyleZhang, Yanjing, Xingyu Cao, Yuyu Zhao, Huahuo Li, Shengwei Xiao, Zhangxin Chen, Guobo Huang, Ye Sun, Zhenzhong Liu, and Zhicai He. 2023. "An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus" Gels 9, no. 4: 279. https://doi.org/10.3390/gels9040279
APA StyleZhang, Y., Cao, X., Zhao, Y., Li, H., Xiao, S., Chen, Z., Huang, G., Sun, Y., Liu, Z., & He, Z. (2023). An Anisotropic Hydrogel by Programmable Ionic Crosslinking for Sequential Two-Stage Actuation under Single Stimulus. Gels, 9(4), 279. https://doi.org/10.3390/gels9040279