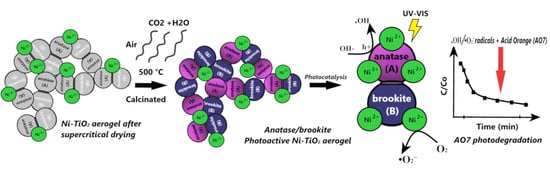
Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystallographic Analysis of Ni, Co, Cu, and Fe-Doped TiO2 Aerogels: Synthesis, Nanostructure, and Calcination Effects
2.1.1. The Influence of Lattice Strain on the Crystalline Size and Photocatalytic Activity of Metal-Doped TiO2 Aerogels
2.1.2. The Role of Dopants on the Crystal Structure and Phase Composition of TiO2
2.2. Effect of Metal-Doped TiO2 Aerogels on the Textural Parameters and Electronic Properties
2.3. Chemical Composition of the Metal-Doped TiO2 Aerogels: Ni, Co, Cu, Fe
2.4. Photoactivity of the Metal-Doped TiO2 Aerogels by Ni, Co, Cu, Fe
2.4.1. Effect of Strain, Cristallynity, and Band Gab over Photocatalytic Performance
2.4.2. Chemical Interaction of the Metal-Doped Aerogels during the Photodegradation of Acid Orange 7
2.4.3. The Impact of Dopant Concentration and Oxygen Vacancies on the Photocatalytic Efficiency of Metal-Doped Aerogel
3. Conclusions
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of the Metal-Doped TiO2 Aerogels by Ni, Co, Cu, Fe
4.3. Characterization of the Metal-Doped TiO2 Aerogels
4.4. Photoactivity Assessment of the Metal-Doped TiO2 Aerogels by Using AO7 as a Model Probe Molecule
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasanpour, M.; Hatami, M. Photocatalytic Performance of Aerogels for Organic Dyes Removal from Wastewaters: Review Study. J. Mol. Liq. 2020, 309, 113094. [Google Scholar] [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Dorraj, M.; Goh, B.T.; Sairi, N.A.; Woi, P.M.; Basirun, W.J. Improved Visible-Light Photocatalytic Activity of TiO2 Co-Doped with Copper and Iodine. Appl. Surf. Sci. 2018, 439, 999–1009. [Google Scholar] [CrossRef]
- Khan, H.; Berk, D. Effect of a Chelating Agent on the Physicochemical Properties of TiO2: Characterization and Photocatalytic Activity. Catal. Letters 2014, 144, 890–904. [Google Scholar] [CrossRef]
- Mandari, K.K.; Police, A.K.R.; Do, J.Y.; Kang, M.; Byon, C. Rare Earth Metal Gd Influenced Defect Sites in N Doped TiO2: Defect Mediated Improved Charge Transfer for Enhanced Photocatalytic Hydrogen Production. Int. J. Hydrog. Energy 2018, 43, 2073–2082. [Google Scholar] [CrossRef]
- Cihlar, J.; Tinoco Navarro, L.K.; Kasparek, V.; Michalicka, J.; Kastyl, J.; Castkova, K.; Celko, L. Influence of LA/Ti Molar Ratio on the Complex Synthesis of Anatase/Brookite Nanoparticles and Their Hydrogen Production. Int. J. Hydrog. Energy 2021, 46, 8578–8593. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.T.; Béland, F.; Do, T.O. Nanocomposite Heterojunctions as Sunlight-Driven Photocatalysts for Hydrogen Production from Water Splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef]
- Cho, H.-W.; Liao, K.-L.; Yang, J.-S.; Wu, J.-J. Revelation of Rutile Phase by Raman Scattering for Enhanced Photoelectrochemical Performance of Hydrothermally-Grown Anatase TiO2 Film. Appl. Surf. Sci. 2018, 440, 125–132. [Google Scholar] [CrossRef]
- Cihlar, J.; Navarro, L.K.T.; Cihlar, J.; Kasparek, V.; Michalicka, J.; Castkova, K.; Lazar, I.; Kastyl, J.; Celko, L.; Vesely, M.; et al. Influence of Substituted Acetic Acids on “Bridge” Synthesis of Highly Photocatalytic Active Heterophase TiO2 in Hydrogen Production. J. Sol.-Gel. Sci. Technol. 2022, 105, 471–488. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Cao, Y.; Xiang, J.; Gao, H. Enhanced Photocatalytic Activities of Low-Bandgap TiO2-Reduced Graphene Oxide Nanocomposites. J. Nanoparticle Res. 2017, 19, 200. [Google Scholar] [CrossRef]
- Hippargi, G.; Mangrulkar, P.; Chilkalwar, A.; Labhsetwar, N.; Rayalu, S. Chloride Ion: A Promising Hole Scavenger for Photocatalytic Hydrogen Generation. Int. J. Hydrog. Energy 2018, 43, 6815–6823. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic Degradation for Environmental Applications—A Review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; García Murillo, A.; Carrillo Romo, F.; Kassiba, A. Effects of Metal Doping (Cu, Ag, Eu) on the Electronic and Optical Behavior of Nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial Hydrogen Production over TiO2-Based Photocatalysts: Polyols, Carboxylic Acids, and Saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Grabowska, E.; Marchelek, M.; Paszkiewicz-Gawron, M.; Zaleska-Medynska, A. Metal Oxide Photocatalysts. In Metal Oxide-Based Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–209. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Wang, L.; Wu, F.; Liu, S.; Chang, C.; Luo, X. A Simple Strategy to Design 3-Layered Au-TiO2 Dual Nanoparticles Immobilized Cellulose Membranes with Enhanced Photocatalytic Activity. Carbohydr. Polym. 2020, 231, 115694. [Google Scholar] [CrossRef]
- Cihlar, J.; Cihlar, J.; Bartonickova, E. Low-Temperature Sol-Gel Synthesis of Anatase Nanoparticles Modified by Au, Pd and Pt and Activity of TiO2/Au, Pd, Pt Photocatalysts in Water Splitting. J. Sol.-Gel. Sci. Technol. 2013, 65, 430–442. [Google Scholar] [CrossRef]
- Cihlar, J.; Vrba, R.; Castkova, K.; Cihlar, J. Effect of Transition Metal on Stability and Activity of La-Ca-M-(Al)-O (M=Co, Cr, Fe and Mn) Perovskite Oxides during Partial Oxidation of Methane. Int. J. Hydrog. Energy 2017, 42, 19920–19934. [Google Scholar] [CrossRef]
- Moradi, V.; Jun, M.B.G.; Blackburn, A.; Herring, R.A. Significant Improvement in Visible Light Photocatalytic Activity of Fe Doped TiO2 Using an Acid Treatment Process. Appl. Surf. Sci. 2018, 427, 791–799. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Gupta, A.K.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Fe-Doped TiO2 Powders for Solar Light Response and Photocatalytic Applications. Process. Appl. Ceram. 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review Doped-TiO2: A Review. Recent Patents Eng. 2014, 2, 157–164. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Li, J.; Yang, X. Photocatalytic Hydrogen Production from Methanol Aqueous Solution under Visible-Light Using Cu/S–TiO2 Prepared by Electroless Plating Method. Catal. Commun. 2015, 59, 189–194. [Google Scholar] [CrossRef]
- Mugundan, S.; Rajamannan, B.; Viruthagiri, G.; Shanmugam, N.; Gobi, R.; Praveen, P. Synthesis and Characterization of Undoped and Cobalt-Doped TiO2 Nanoparticles via Sol–Gel Technique. Appl. Nanosci. 2015, 5, 449–456. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt Doped TiO2: A Stable and Efficient Photocatalyst for Continuous Hydrogen Production from Glycerol: Water Mixtures under Solar Light Irradiation. Int. J. Hydrog. Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Seadira, T.; Sadanandam, G.; Ntho, T.A.; Lu, X.; Masuku, C.M.; Scurrell, M. Hydrogen Production from Glycerol Reforming: Conventional and Green Production. Rev. Chem. Eng. 2018, 34, 695–726. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Gomez, M.J.; Lacconi, G.I. One Step Synthesis of High Efficiency Nickel/Mesoporous TiO2 Hybrid Catalyst for Hydrogen Evolution Reaction. J. Energy Chem. 2019, 29, 79–87. [Google Scholar] [CrossRef]
- Lamouchi, W.; Ben Slama, S.; Saadallah, F.; Bouaicha, M. Nickel Doping Induced Amorphization of Brookite TiO2: Photoluminescence Enhancement. Optik 2021, 227, 166123. [Google Scholar] [CrossRef]
- Ali, H.; Vandevyvere, T.; Lauwaert, J.; Kansal, S.K.; Saravanamurugan, S.; Thybaut, J.W. Impact of Oxygen Vacancies in Ni Supported Mixed Oxide Catalysts on Anisole Hydrodeoxygenation. Catal. Commun. 2022, 164, 106436. [Google Scholar] [CrossRef]
- Moussaoui, R.; Elghniji, K.; ben Mosbah, M.; Elaloui, E.; Moussaoui, Y. Sol–Gel Synthesis of Highly TiO2 Aerogel Photocatalyst via High Temperature Supercritical Drying. J. Saudi Chem. Soc. 2017, 21, 751–760. [Google Scholar] [CrossRef]
- Budtova, T.; Aguilera, D.A.; Beluns, S.; Berglund, L.; Chartier, C.; Espinosa, E.; Gaidukovs, S.; Klimek-kopyra, A.; Kmita, A.; Lachowicz, D.; et al. Biorefinery Approach for Aerogels. Polymers 2020, 12, 2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, C.; Shi, Z.; Zhi, M.; Hong, Z. The Investigation of an Organic Acid Assisted Sol-Gel Method for Preparing Monolithic Zirconia Aerogels. RSC Adv. 2018, 8, 8011–8020. [Google Scholar] [CrossRef] [PubMed]
- Vorsina, I.; Grigorieva, T.; Barinova, A.; Lyakhov, N. Mechanochemical Interaction of Silicon Dioxide With Organic Acids. Chem. Sustain. Dev. 2011, 19, 236–247. [Google Scholar]
- Cihlar, J.; Kasparek, V.; Kralova, M.; Castkova, K. Biphasic Anatase-Brookite Nanoparticles Prepared by Sol-Gel Complex Synthesis and Their Photocatalytic Activity in Hydrogen Production. Int. J. Hydrog. Energy 2015, 40, 2950–2962. [Google Scholar] [CrossRef]
- Mahy, J.G.; Cerfontaine, V.; Poelman, D.; Devred, F.; Gaigneaux, E.M.; Heinrichs, B.; Lambert, S.D. Highly Efficient Low-Temperature N-Doped TiO2 Catalysts for Visible Light Photocatalytic Applications. Materials 2018, 11, 584. [Google Scholar] [CrossRef]
- Lal, M.; Sharma, P.; Ram, C. Calcination Temperature Effect on Titanium Oxide (TiO2) Nanoparticles Synthesis. Optik 2021, 241, 166934. [Google Scholar] [CrossRef]
- Kim, M.G.; Kang, J.M.; Lee, J.E.; Kim, K.S.; Kim, K.H.; Cho, M.; Lee, S.G. Effects of Calcination Temperature on the Phase Composition, Photocatalytic Degradation, and Virucidal Activities of TiO2Nanoparticles. ACS Omega 2021, 6, 10668–10678. [Google Scholar] [CrossRef]
- Kim, G.H.; Jeong, S. Change of Electronic Structures by Dopant-Induced Local Strain. Sci. Rep. 2015, 5, 11227. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Z.; Chen, J.; Sheng, W.; Sun, L.; Xiang, J. Effect of Calcination Temperature on the Activity and Structure of MnOx/TiO2 Adsorbent for Hg0 Removal. Fuel Process. Technol. 2015, 135, 25–33. [Google Scholar] [CrossRef]
- Espinos, J.P.; Gonz, A.R.; Caballero, A.; GARCfA, J.; Munuera, G. The State of Nickel in Ni/SiO2 and Ni/TiO2-Calcined Catalysts. J. Catal. 1992, 136, 415–422. [Google Scholar] [CrossRef]
- Tyo, E.C.; Yin, C.; Di Vece, M.; Qian, Q.; Kwon, G.; Lee, S.; Lee, B.; Debartolo, J.E.; Seifert, S.; Winans, R.E.; et al. Oxidative Dehydrogenation of Cyclohexane on Cobalt Oxide (Co3O4) Nanoparticles: The Effect of Particle Size on Activity and Selectivity. ACS Catal. 2012, 2, 2409–2423. [Google Scholar] [CrossRef]
- Habibi, M.H.; Shojaee, E. Synthesis of a Heterojunction CoTiO3/Co3O4 Nano-Composite Thin Film with Superior Photocatalytic Activity and Reusability: Effect of Calcination Temperature on Phase Transformation and Effect of Oxidants on Enhanced Degradation of Indo Light Blue Dye. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 229, 117796. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi Miyamoto, N.; Miyamoto, R.; Giamello, E.; Kurisaki, T.; Wakita, H. Evaluation of Coexistent Metal Ions with TiO2: An EPR Approach. Res. Chem. Intermed. 2018, 44, 4563–4575. [Google Scholar] [CrossRef]
- Phani, A.R.; Santucci, S. Structural Characterization of Nickel Titanium Oxide Synthesized by Sol-Gel Spin Coating Technique. Thin Solid Films 2001, 396, 1–4. [Google Scholar] [CrossRef]
- Gabal, M.A.E.F.; Al Angari, Y.M.; Obaid, A.Y. Structural Characterization and Activation Energy of NiTiO3 Nanopowders Prepared by the Co-Precipitation and Impregnation with Calcinations. Comptes Rendus Chim. 2013, 16, 704–711. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Amal, R.; Teoh, W.Y.; Kulak, A.I. Studies of Nanosized Iron-Doped TiO2 Photocatalysts by Spectroscopic Methods. Appl. Magn. Reson. 2017, 48, 447–459. [Google Scholar] [CrossRef]
- Fernando, N.; Swaminathan, J.; Robles Hernandez, F.C.; Priyadarshana, G.; Sandaruwan, C.; Yang, W.; Karunaratne, V.; Wang, Z.; Amaratunga, G.A.J.; Kottegoda, N.; et al. Pseudobrookite Based Heterostructures for Efficient Electrocatalytic Hydrogen Evolution. Mater. Rep. Energy 2021, 1, 100020. [Google Scholar] [CrossRef]
- Dubey, R.S.; Jadkar, S.R.; Bhorde, A.B. Synthesis and Characterization of Various Doped TiO2 Nanocrystals for Dye-Sensitized Solar Cells. ACS Omega 2021, 6, 3470–3482. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Llewellyn, P.; Maurin, G.; Sing, K.S.W. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications. Available online: https://books.google.cz/books?hl=es&lr=&id=UOE-ZscCYncC&oi=fnd&pg=PP1&ots=0T__DEslnv&sig=jF4A7rVRX5_aDPQtMEamWcE7XvM&redir_esc=y#v=onepage&q&f=false (accessed on 9 November 2022).
- Yu, J.; Yu, H.; Cheng, B.; Trapalis, C. Effects of Calcination Temperature on the Microstructures and Photocatalytic Activity of Titanate Nanotubes. J. Mol. Catal. A Chem. 2006, 249, 135–142. [Google Scholar] [CrossRef]
- Wu, S.; Ishisone, K.; Sheng, Y.; Manuputty, M.Y.; Kraft, M.; Xu, R. TiO2 with Controllable Oxygen Vacancies for Efficient Isopropanol Degradation: Photoactivity and Reaction Mechanism. Catal. Sci. Technol. 2021, 11, 4060–4071. [Google Scholar] [CrossRef]
- Singh, R.; Dutta, S. Synthesis and Characterization of Solar Photoactive TiO2 Nanoparticles with Enhanced Structural and Optical Properties. Adv. Powder Technol. 2018, 29, 211–219. [Google Scholar] [CrossRef]
- Rahul, T.K.; Mohan, M.; Sandhyarani, N. Enhanced Solar Hydrogen Evolution over in Situ Gold-Platinum Bimetallic Nanoparticle-Loaded Ti3+ Self-Doped Titania Photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 3049–3059. [Google Scholar] [CrossRef]
- Qiu, C.; Lin, J.; Shen, J.; Liu, D.; Zhang, Z.; Lin, H.; Wang, X. Regulation of the Rutile/Anatase TiO2 heterophase Interface by Ni12P5to Improve Photocatalytic Hydrogen Evolution. Catal. Sci. Technol. 2020, 10, 3709–3719. [Google Scholar] [CrossRef]
- Liu, J.; Hodes, G.; Yan, J.; Liu, S. (Frank) Metal-Doped Mo2C (Metal = Fe, Co, Ni, Cu) as Catalysts on TiO2 for Photocatalytic Hydrogen Evolution in Neutral Solution. Chinese J. Catal. 2020, 42, 205–216. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.N.; Kumar, R. Formation of Oxygen Vacancies and Ti3+ State in TiO2 Thin Film and Enhanced Optical Properties by Air Plasma Treatment. Sci. Rep. 2016, 6, srep32355. [Google Scholar] [CrossRef] [PubMed]
- Lyson-Sypien, B.; Czapla, A.; Zakrzewska, K.; Swierczek, K.; Radecka, M.; Rekas, M.; Michalow, K.; Graule, T. Influence of Grain Size on Gas Sensing Properties of TiO2 Nanopowders. Procedia Eng. 2012, 47, 1057–1060. [Google Scholar] [CrossRef]
- Godiksen, A.; Stappen, F.N.; Vennestrøm, P.N.R.; Giordanino, F.; Birk Rasmussen, S.; Lundegaard, L.F.; Mossin, S.; Topsøe, H.A. Coordination Environment of Copper Sites in Cu-CHA Zeolite Investigated by Electron Paramagnetic Resonance. J. Phys. Chem. C 2014, 118, 23126–23138. [Google Scholar] [CrossRef]
- Zhou, S.; Cižmár, E.; Potzger, K.; Krause, M.; Talut, G.; Helm, M.; Fassbender, J.; Zvyagin, S.A.; Wosnitza, J.; Schmidt, H. Origin of Magnetic Moments in Defective TiO2 Single Crystals. Phys. Rev. B 2009, 79, 113201. [Google Scholar] [CrossRef]
- Niemöller, A.; Jakes, P.; Eurich, S.; Paulus, A.; Kungl, H.; Eichel, R.-A.; Granwehr, J.; Niemöller, N.; Eichel, R.-A. Monitoring Local Redox Processes in LiNi0.5Mn1.5O4 Battery Cathode Material by in Operando EPR Spectroscopy. Cit. J. Chem. Phys. 2018, 148, 14705. [Google Scholar] [CrossRef]
- Savoyant, A.; Alnoor, H.; Bertaina, S.; Nur, O.; Willander, M. EPR Investigation of Pure and Co-Doped ZnO Oriented Nanocrystals. Nanotechnology 2017, 28, 035705. [Google Scholar] [CrossRef]
- Bennett, B.; Kowalski, J.M. EPR Methods for Biological Cu(II): L-Band CW and NARS. Methods Enzymol. 2015, 563, 341. [Google Scholar] [CrossRef]
- Kumar, C.P.; Gopal, N.O.; Wang, T.C.; Wong, M.S.; Ke, S.C. EPR Investigation of TiO2 Nanoparticles with Temperature-Dependent Properties. J. Phys. Chem. B 2006, 110, 5223–5229. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shahwar, D.; Khatoon, B. Trans-Generational Response of TiO2 Nanoparticles in Inducing Variability and Changes in Biochemical Pool of Lentil F2 Progenies. J. Biosci. 2022, 47, 35. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, H.A.; Narasimharao, K.; Ali, T.T.; Khalil, K.M.S. Acidic Peptizing Agent Effect on Anatase-Rutile Ratio and Photocatalytic Performance of TiO2 Nanoparticles. Nanoscale Res. Lett. 2018, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Chelbi, S.; Djouadi, D.; Chelouche, A.; Hammiche, L.; Touam, T.; Doghmane, A. Effects of Ti-Precursor Concentration and Annealing Temperature on Structural and Morphological Properties of TiO2 Nano-Aerogels Synthesized in Supercritical Ethanol. SN Appl. Sci. 2020, 2, 872. [Google Scholar] [CrossRef]
- Asenjo, N.G.; Santamaría, R.; Blanco, C.; Granda, M.; Álvarez, P.; Menéndez, R. Correct Use of the Langmuir–Hinshelwood Equation for Proving the Absence of a Synergy Effect in the Photocatalytic Degradation of Phenol on a Suspended Mixture of Titania and Activated Carbon. Carbon 2013, 55, 62–69. [Google Scholar] [CrossRef]
- Qourzal, S.; Barka, N.; Tamimi, M.; Assabbane, A.; Ait-Ichou, Y. Photodegradation of 2-Naphthol in Water by Artificial Light Illumination Using TiO2 Photocatalyst: Identification of Intermediates and the Reaction Pathway. Appl. Catal. A Gen. 2008, 334, 386–393. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band Gap Engineered TiO2 Nanoparticles for Visible Light Induced Photoelectrochemical and Photocatalytic Studies. J. Mater. Chem. A 2014, 2, 637–644. [Google Scholar] [CrossRef]
- Jafarikojour, M.; Dabir, B.; Sohrabi, M.; Royaee, S.J. Evaluation and Optimization of a New Design Photocatalytic Reactor Using Impinging Jet Stream on a TiO2 Coated Disc. Chem. Eng. Process. Process. Intensif. 2017, 121, 215–223. [Google Scholar] [CrossRef]
- Phromma, S.; Wutikhun, T.; Kasamechonchung, P.; Eksangsri, T.; Sapcharoenkun, C. Effect of Calcination Temperature on Photocatalytic Activity of Synthesized TiO2 Nanoparticles via Wet Ball Milling Sol-Gel Method. Appl. Sci. 2020, 10, 993. [Google Scholar] [CrossRef]
- Muthee, D.K.; Dejene, B.F. Effect of Annealing Temperature on Structural, Optical, and Photocatalytic Properties of Titanium Dioxide Nanoparticles. Heliyon 2021, 7, e07269. [Google Scholar] [CrossRef]
- Ganesh, I.; Gupta, A.K.; Kumar, P.P.; Sekhar, P.S.C.; Radha, K.; Padmanabham, G.; Sundararajan, G. Preparation and Characterization of Ni-Doped TiO2 Materials for Photocurrent and Photocatalytic Applications. Sci. World J. 2012, 2012, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, D.D.; Li, G.; Wang, Y. Investigation of the Roles of Active Oxygen Species in Photodegradation of Azo Dye AO7 in TiO2 Photocatalysis Illuminated by Microwave Electrodeless Lamp. J. Photochem. Photobiol. A Chem. 2008, 199, 311–315. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, e1807660. [Google Scholar] [CrossRef] [PubMed]
- Torres-Rodríguez, J.; Kalmár, J.; Menelaou, M.; Čelko, L.; Dvořak, K.; Cihlář, J.; Cihlař, J.; Kaiser, J.; Győri, E.; Veres, P.; et al. Heat Treatment Induced Phase Transformations in Zirconia and Yttria-Stabilized Zirconia Monolithic Aerogels. J. Supercrit. Fluids 2019, 149, 54–63. [Google Scholar] [CrossRef]














| Aerogel Sample | Crystal Phases | Content of Phases (wt.%) | The Crystallite Size (nm) | Lattice Strain (%) | Lattice Parameters (nm) | ||
|---|---|---|---|---|---|---|---|
| a | b | c | |||||
| Temperature of calcination 500 °C | |||||||
| TiO2 | A | 99.08 | 11.0 | 0.4 | 0.3779 | 0.3779 | 0.9450 |
| R | 0.92 | 8.6 | 0 | 0.4540 | 0.4540 | 0.3030 | |
| Ni–TiO2 | A | 75.5 | 5.0 | 0.6 | 0.3798 | 0.3798 | 0.9509 |
| B | 24 | 2.0 | 2.3 | 0.9313 | 0.5433 | 0.5308 | |
| NiTiO3 | 0.6 | 3.2 | 1.3 | 0.4367 | 0.4367 | 0.1253 | |
| Co–TiO2 | A | 79 | 7.1 | 0.8 | 0.3770 | 0.3770 | 0.9390 |
| B | 20 | 2.2 | 2.5 | 0.937 | 0.5446 | 0.5357 | |
| Co3O4 | 1.0 | 2.3 | 0.52 | 0.7054 | 0.7054 | 0.7054 | |
| Cu–TiO2 | A | 77.2 | 7.0 | 0.4 | 0.3784 | 0.3784 | 0.9400 |
| B | 22 | 3.0 | 1.3 | 0.907 | 0.566 | 0.5300 | |
| Cu3TiO4 | 0.8 | 5.0 | 0.74 | 0.319 | 0.319 | 0.1030 | |
| Fe–TiO2 | A | 82 | 4.4 | 0.4 | 0.3778 | 0.3778 | 0.9410 |
| B | 16 | 3.0 | 1.24 | 0.9540 | 0.5582 | 0.4768 | |
| FeTiO3 | 2.0 | 2.0 | 2.7 | 0.2482 | 0.9830 | 0.6180 | |
| Temperature of calcination 900 °C | |||||||
| TiO2 | R | 100 | 66.2 | 0 | 0.4603 | 0.4603 | 0.2967 |
| Ni–TiO2 | R | 98 | 72.3 | 0 | 0.4602 | 0.4602 | 0.2966 |
| NiTiO3 | 2 | 15.3 | 0.35 | 0.5036 | 0.5036 | 1.3814 | |
| Co–TiO2 | R | 95 | 69.0 | 0 | 0.4599 | 0.4599 | 0.29643 |
| CoTiO3 | 5 | 20.0 | 0.25 | 0.5066 | 0.5066 | 1.3928 | |
| Cu–TiO2 | A | 2.2 | 10.1 | 0.45 | 0.3852 | 0.3852 | 0.9806 |
| R | 93 | 78.0 | 0 | 0.4596 | 0.4596 | 0.2962 | |
| CuO | 4.8 | 3.3 | 1.5 | 0.3884 | 0.2879 | 0.559 | |
| Fe–TiO2 | R | 87.3 | 67.0 | 0.01 | 0.4602 | 0.4602 | 0.2966 |
| Fe2TiO5 | 12.7 | 36.4 | 0.14 | 0.9803 | 1.0002 | 0.3729 | |
| Material | B/A Ratio | BET SSA (m2·g−1) | BJH Pore Size (nm) | BJH Pore Volume (cm3/g−1) | Band Gap (eV) |
|---|---|---|---|---|---|
| TiO2 aerogel | 0 | 86.5 | 32.1 | 0.79 | 3.0 |
| Ni–TiO2 aerogel | 0.31 | 129.3 | 17.4 | 0.82 | 2.1 |
| Co–TiO2 aerogel | 0.25 | 115.7 | 17.5 | 0.80 | 2.4 |
| Cu–TiO2 aerogel | 0.28 | 157.6 | 17.2 | 0.98 | 2.3 |
| Fe–TiO2 aerogel | 0.19 | 158.1 | 12.2 | 0.73 | 1.7 |
| Element | Ti | O | (Ni, Co, Cu, and Fe) | |||
|---|---|---|---|---|---|---|
| Material | Wt.% | At% | Wt.% | At% | Wt.% | At% |
| Ni–TiO2 aerogel | 61.6 | 35 | 37.7 | 64.7 | 0.7 | 0.3 |
| Co–TiO2 aerogel | 55.6 | 29.6 | 43.7 | 70.1 | 0.7 | 0.3 |
| Cu–TiO2 aerogel | 52.2 | 27.3 | 45.9 | 72 | 1.9 | 0.7 |
| Fe–TiO2 aerogel | 42.9 | 21.1 | 52.2 | 76.9 | 4.9 | 2.1 |
| Aerogel Samples | TiO2 | Ni–TiO2 | Co–TiO2 | Cu–TiO2 | Fe–TiO2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elements | Wt.% | At.% | Wt.% | At.% | Wt.% | At.% | Wt.% | At.% | Wt.% | At.% |
| C 1s (285.5 eV) | 6.70 | 13.55 | 6.45 | 13.02 | 5.78 | 11.9 | 10.3 | 19.7 | 6.95 | 13.9 |
| Ti 2p (458.7–464.5 eV) | 54.62 | 27.72 | 52.89 | 26.79 | 54.98 | 28.5 | 48.2 | 23.1 | 47.95 | 24.04 |
| O 1s (530.2 eV) | 38.67 | 58.72 | 39.37 | 59.66 | 38.09 | 59.1 | 39.24 | 56.3 | 39.9 | 59.8 |
| Ni, Co, Cu, and Fe 2p (861.4 eV, 787.2, 933.9 eV, 711.3) | - | - | 1.29 | 0.53 | 1.16 | 0.49 | 2.21 | 0.8 | 5.27 | 2.27 |
| Aerogel | B/A | SSA (m2.g−1) | Band Gap (eV) | Dopant wt.% | Kapp (min−1) |
|---|---|---|---|---|---|
| TiO2 aerogel | 0 | 86.5 | 3.0 | 0 | 4.0 × 10−4 |
| Ni–TiO2 aerogel | 0.31 | 129.3 | 2.1 | 1.0 | 2.0 × 10−3 |
| Co–TiO2 aerogel | 0.25 | 115.7 | 2.4 | 1.0 | 6.0 × 10−4 |
| Cu–TiO2 aerogel | 0.28 | 157.6 | 2.3 | 2.0 | 1.6 × 10−3 |
| Fe–TiO2 aerogel | 0.19 | 158.1 | 1.7 | 5.0 | 7.0 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lizeth Katherine, T.N.; Vendula, B.; Jaroslav, K.; Jaroslav, C. Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels. Gels 2023, 9, 357. https://doi.org/10.3390/gels9050357
Lizeth Katherine TN, Vendula B, Jaroslav K, Jaroslav C. Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels. Gels. 2023; 9(5):357. https://doi.org/10.3390/gels9050357
Chicago/Turabian StyleLizeth Katherine, Tinoco Navarro, Bednarikova Vendula, Kastyl Jaroslav, and Cihlar Jaroslav. 2023. "Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels" Gels 9, no. 5: 357. https://doi.org/10.3390/gels9050357
APA StyleLizeth Katherine, T. N., Vendula, B., Jaroslav, K., & Jaroslav, C. (2023). Structure and Photocatalytic Properties of Ni-, Co-, Cu-, and Fe-Doped TiO2 Aerogels. Gels, 9(5), 357. https://doi.org/10.3390/gels9050357