Hybrid Cryogels with Superabsorbent Properties as Promising Materials for Penicillin G Retention
Abstract
:1. Introduction
2. Results and Discussion
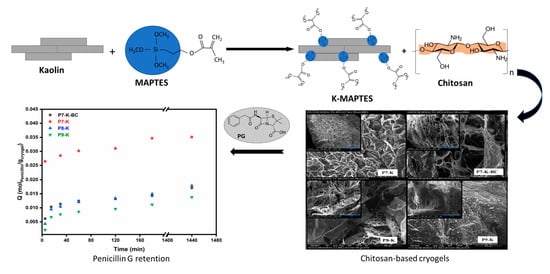
2.1. Synthesis of Hybrid Cryogels
2.1.1. Fourier Transform Infrared Spectrometry (FTIR)
2.1.2. Thermogravimetric Analysis (TGA/DTG)
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Nitrogen Intrusion Porosimetry, Brunauer–Emmett–Teller (BET)
2.1.5. Determination of the Swelling Degrees (SDs)
2.1.6. Evaluation of PG Retention by Batch Adsorption Measurements
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Hybrid Cryogels Based on Chitosan, Biocellulose, and Kaolin
4.2.1. Modification of K with MAPTES Coupling Agent
4.2.2. Preparation of Hybrid Cryogels
4.3. Characterization Techniques
4.4. Determination of Swelling Degrees (SD)
4.5. Retention Capacity of Cryogels for Penicillin G, as Model Antibiotic
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, B. Water pollution by agriculture. Phil. Trans. R. Soc. B 2008, 363, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Graham, D.W.; Sreekrishnan, T.R.; Ahammad, S.Z. Heavy metal and antibiotic resistance in four Indian and UK rivers with different levels and types of water pollution. Sci. Total Environ. 2023, 857, 159059. [Google Scholar] [CrossRef]
- Pouramini, Z.; Mousavi, S.M.; Babapoor, A.; Hashemi, S.A.; Lai, C.W.; Mazaheri, Y.; Chiang, W.-H. Effect of Metal Atom in Zeolitic Imidazolate Frameworks (ZIF-8 & 67) for Removal of Dyes and Antibiotics from Wastewater: A Review. Catalysts 2023, 13, 155. [Google Scholar]
- Zhang, X.; Li, F.; Zhao, X.; Cao, J.; Liu, S.; Zhang, Y.; Yuan, Z.; Huang, X.; Hoop, C.F.; Peng, X.; et al. Bamboo Nanocellulose/Montmorillonite Nanosheets/Polyethyleneimine Gel Adsorbent for Methylene Blue and Cu(II) Removal from Aqueous Solutions. Gels 2023, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Bangari, R.S.; Sinha, N. Adsorption of tetracycline, ofloxacin and cephalexin antibiotics on boron nitride nanosheets from aqueous solution. J. Mol. Liq. 2019, 293, 111376. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Urraca, J.L.; Hall, A.J.; Moreno-Bondi, M.C.; Sellergren, B. A Stoichiometric Molecularly Imprinted Polymer for the Class-Selective Recognition of Antibiotics in Aqueous Media. Angew. Chem. Int. Ed. 2006, 45, 5158–5161. [Google Scholar] [CrossRef]
- Li, D.; Yang, M.; Hu, J.; Zhang, Y.; Chang, H.; Jin, F. Determination of penicillin G and its degradation products in a penicillin production wastewater treatment plant and the receiving river. Water Res. 2008, 42, 307–317. [Google Scholar] [CrossRef]
- Raichur, A.; Sinha, N. Synthesis of multi-layered nanoswabs for simultaneous and expeditious removal of antibiotic-resistant bacteria, dyes, and antibiotics from wastewater. Sep. Purif. Tehnol. 2023, 308, 122830. [Google Scholar] [CrossRef]
- Kuhn, J.; Aylaz, G.; Sari, E.; Marco, M.; Yiu, H.H.P.; Duman, M. Selective binding of antibiotics using magnetic molecularly imprinted polymer (MMIP) networks prepared from vinyl-functionalized magnetic nanoparticles. J. Hazard. Mater. 2020, 387, 121709. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Watkinson, A.J.; Murby, E.J.; Costanzo, S.D. Removal of antibiotics in conventional and advanced wastewater treatment: Implications for environmental discharge and wastewater recycling. Water Res. 2007, 41, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Polo, M.; Velo-Gala, I.; López-Peñalver, J.J.; Rivera-Utrilla, J. Molecular imprinted polymer to remove tetracycline from aqueous solutions. Microporous Mesoporous Mater. 2015, 203, 32–40. [Google Scholar] [CrossRef]
- Ambreen, J.; Haleem, A.; Shah, A.A.; Mushtaq, F.; Siddiq, M.; Bhatti, M.A.; Bukhari, S.N.U.S.; Chandio, A.D.; Mahdi, W.A.; Alshehri, S. Facile Synthesis and Fabrication of NIPAM-Based Cryogels for Environmental Remediation. Gels 2023, 9, 64. [Google Scholar] [CrossRef]
- Wang, C.; Yao, Z.; Zhan, P.; Yi, X.; Chen, J.; Xiong, J. Significant tipping points of sediment microeukaryotes forewarn increasing antibiotic pollution. J. Environ. Sci. 2023, 124, 429–439. [Google Scholar] [CrossRef]
- Rashed, A.O.; Huynh, C.; Merenda, A.; Qin, S.; Usman, K.A.S.; Sadek, A.; Kong, L.; Kondo, T.; Dumée, L.F.; Razal, J.M. Schottky-like photo/electro-catalytic carbon nanotube composite ultrafiltration membrane reactors. Carbon 2023, 204, 238–253. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Q. Algal fouling of microfiltration and ultrafiltration membranes and control strategies: A review. Sep. Purif. Technol. 2018, 203, 193–208. [Google Scholar] [CrossRef]
- Yeşilova, E.; Osman, B.; Kara, A.; Özer, E.T. Molecularly imprinted particle embedded composite cryogel for selective tetracycline adsorption. Sep. Purif. Technol. 2018, 200, 155–163. [Google Scholar] [CrossRef]
- Kammakakam, I.; Lai, Z. Next-generation ultrafiltration membranes: A review of material design, properties, recent progress, and challenges. Chemosphere 2023, 316, 137699. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose Cryogels as Promising Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2037. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I. Cryostructuring of Polymeric Systems. 50. Cryogels and Cryotropic Gel-Formation: Terms and Definitions. Gels 2018, 4, 77. [Google Scholar] [CrossRef]
- Jones, L.O.; Williams, L.; Boam, T.; Kalmet, M.; Oguike, C.; Hatton, F.L. Cryogels: Recent applications in 3D-bioprinting, injectable cryogels, drug delivery, and wound healing. Beilstein J. Org. Chem. 2021, 17, 2553–2569. [Google Scholar] [CrossRef] [PubMed]
- Hixon, K.R.; Lu, T.; Sell, S.A. A comprehensive review of cryogels and their roles in tissue engineering applications. Acta Biomater. 2017, 62, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Kemençe, N.; Bölgen, N. Gelatin-and hydroxyapatite-based cryogels for bone tissue engineering: Synthesis, characterization, in vitro and in vivo biocompatibility. J. Tissue Eng. Regen. Med. 2017, 11, 20–33. [Google Scholar] [CrossRef]
- Korhonen, O.; Budtova, T. All-cellulose composite aerogels and cryogels. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106027. [Google Scholar] [CrossRef]
- Buchtová, N.; Pradille, C.; Bouvard, J.-L.; Budtova, T. Mechanical properties of cellulose aerogels and cryogels. Soft Matter 2019, 15, 7901–7908. [Google Scholar] [CrossRef]
- Eggermont, L.J.; Rogers, Z.J.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable cryogels for biomedical applications. Trends Biotechnol. 2020, 38, 418–431. [Google Scholar] [CrossRef]
- Baimenov, A.; Berillo, D.A.; Poulopoulos, S.G.; Inglezakis, V.J. A review of cryogels synthesis, characterization and applications on the removal of heavy metals from aqueous solutions. Adv. Colloid Interface Sci. 2020, 276, 102088. [Google Scholar] [CrossRef]
- Saylan, Y.; Denizli, A. Supermacroporous composite cryogels in biomedical applications. Gels 2019, 5, 20. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Chen, L.; Dai, B. Control of ice crystal growth and its effect on porous structure of chitosan cryogels. Chem. Eng. Sci. 2019, 201, 50–57. [Google Scholar] [CrossRef]
- Evans, C.; Morimitsu, Y.; Hisadome, T.; Inomoto, F.; Yoshida, M.; Takei, T. Optimized hydrophobically modified chitosan cryogels for strength and drug delivery systems. J. Biosci. Bioeng. 2021, 132, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Dahman, Y.; Jayasuriya, K.E.; Kalis, M. A Potential of Biocellulose Nanofibers Production from Agricultural Renewable Resources: Preliminary Study. Appl. Biochem. Biotechnol. 2010, 162, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Burkatovskaya, M.; Tegos, G.P.; Swietlik, E.; Demidova, T.N.; Castano, A.P.; Hamblin, M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials 2006, 27, 4157–4164. [Google Scholar] [CrossRef]
- Krishnaveni, B.; Ragunathan, R. Extraction and Characterization of Chitin and Chitosan from F.solani CBNR BKRR, Synthesis of their Bionanocomposites and Study of their Productive Application. J. Pharm. Sci. Res. 2015, 7, 197–205. [Google Scholar]
- Piacham, T.; Isarankura-Na-Ayudhya, C.; Prachayasittikul, V. A simple method for creating molecularly imprinted polymer-coated bacterial cellulose nanofibers. Chem. Pap. 2014, 68, 838–841. [Google Scholar] [CrossRef]
- Yilmaz, C.N.C.; Yilmaz, O.; Kose, F.A.; Bibire, N. Chitosan-Graft-Poly(N-Isopropylacrylamide)/PVA Cryogels as Carriers for Mucosal Delivery of Voriconazole. Polymers 2019, 11, 1432. [Google Scholar] [CrossRef]
- Liu, X.; Peng, W.; Wang, Y.; Zhu, M.; Sun, T.; Peng, Q.; Zeng, Y.; Feng, B.; Lu, X.; Weng, J.; et al. Synthesis of an RGD-grafted oxidized sodium alginate–N-succinyl chitosan hydrogel and an in vitro study of endothelial and osteogenic differentiation. J. Mater. Chem. B 2013, 1, 4484–4492. [Google Scholar] [CrossRef]
- Bakadia, B.M.; Boni, B.; Ahmed, A.; Zheng, R.; Shi, Z.; Ullah, M.W.; Lamboni, L.; Yang, G. In situ synthesized porous bacterial cellulose/poly(vinyl alcohol)-based silk sericin and azithromycin release system for treating chronic wound biofilm. Macromol. Biosci. 2022, 22, 2200201. [Google Scholar] [CrossRef]
- Ullah, H.; Wahid, F.; Santos, H.A.; Khan, T. Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr. Polym. 2016, 150, 330–352. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Stilwell, M.D.; Santos, T.M.A.; Wang, H.; Weibel, D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015, 12, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, C.; Peng, Z.; Zhang, S. Characterization and thermal behavior of kaolin. J. Therm. Anal. Calorim. 2011, 105, 157–160. [Google Scholar] [CrossRef]
- Nigam, V.; Lal, G. Review on Recent Trends in Polymer Layered Clay Nanocomposites. Proc. Indian Natl. Sci. Acad. 2008, 74, 87–96. [Google Scholar]
- Shanmugharaj, A.M.; Rhee, K.Y.; Ryuet, S.H. Influence of Dispersing Medium on Grafting of Aminopropyltriethoxysilane in Swelling Clay Materials. J. Colloid Interface Sci. 2006, 298, 854–859. [Google Scholar] [CrossRef]
- Tămășan, M.; Simion, V. Thermal and structural characterization of polyvinyl alcohol- kaolinite nanocomposites. Dig. J. Nanomater. Biostruct. 2011, 6, 1311–1316. [Google Scholar]
- Tian, L.; Abukhadra, R.M.; Mohamed, A.S.; Nadeem, A.; Ahmad, S.F.; Ibrahim, E.K. Insight into the Loading and Release Properties of an Exfoliated Kaolinite/Cellulose Fiber (EXK/CF) Composite as a Carrier for Oxaliplatin Drug: Cytotoxicity and Release Kinetics. ACS Omega 2020, 5, 19165–19173. [Google Scholar] [CrossRef]
- Dumitru, M.V.; Sandu, T.; Ciurlică, A.L.; Neblea, I.E.; Trică, B.; Ghebaur, A.; Gârea, S.A.; Horia, I.; Sârbu, A.; Iordache, T.V. Organically modified montmorillonite as pH versatile carriers for delivery of 5-aminosalicylic acid. Appl. Clay Sci. 2022, 218, 106415. [Google Scholar] [CrossRef]
- Zaharia, A.; Perrin, F.-X.; Teodorescu, M.; Radu, A.-L.; Iordache, T.-V.; Florea, A.-M.; Donescu, D.; Sarbu, A. New organophilic kaolin clays based on single-point grafted 3-aminopropyl dimethylethoxysilane. Phys. Chem. Chem. Phys. 2015, 17, 24908. [Google Scholar] [CrossRef]
- Reddy, O.S.; Subha, M.C.S.; Jithendra, T.; Madhavi, C.; Rao, K.C.; Mallikarjuna, B. Sodium Alginate/Gelatin Microbeads-Intercalated with Kaolin Nanoclay for Emerging Drug Delivery in Wilson’s Disease. Int. J. Appl. Pharm. 2019, 11, 71–80. [Google Scholar] [CrossRef]
- Carvalho, A.J.F.; Curvelo, A.A.S.; Agnelli, J.A.M. A first insight on composites of thermoplastic starch and kaolin. Carbohydr. Polym. 2001, 45, 189–194. [Google Scholar] [CrossRef]
- Mucsi, Z.; Chass, A.G.; Balogh, A.P.; Jojart, B.; Fang, D.K.; Cuesta, R.J.A.; Viskolcz, B.; Csizmadia, G. Penicillin’s catalytic mechanism revealed by inelastic neutrons and quantum chemical theory. Phys. Chem. Chem. Phys. 2013, 15, 20447–20455. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.; Sarbu, A.; Zaharia, A.; Sandu, T.; Iovu, H.; Fierascu, R.C.; Neagu, A.-L.; Chiriac, A.-L.; Iordache, T.-V. A Top-Down Procedure for Synthesizing Calcium Carbonate-Enriched Chitosan from Shrimp Shell Wastes. Gels 2022, 8, 742. [Google Scholar] [CrossRef] [PubMed]
- Frone, A.N.; Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R.; Casarica, A.; Stanescu, P.O.; Baciu, D.D.; Salageanu, A. Bacterial cellulose sponges obtained with green cross-linkers for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110740. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ueda, M.; Sugikawa, K.; Yasuhara, K.; Ikeda, A. Light-triggered hydrophilic drug release from liposomes through removal of a photolabile protecting group. RSC Adv. 2019, 9, 166. [Google Scholar] [CrossRef]
- Balan, E.; Saita, A.M.; Mauri, F.; Calas, G. First-principles modeling of the infrared spectrum of kaolinite. Am. Mineral. 2001, 86, 1321–1330. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Neblea, I.E.; Chiriac, A.-L.; Zaharia, A.; Sarbu, A.; Teodorescu, M.; Miron, A.; Paruch, L.; Paruch, A.M.; Olaru, A.G.; Iordache, T.-V. Introducing Semi-Interpenetrating Networks of Chitosan and Ammonium-Quaternary Polymers for the Effective Removal of Waterborne Pathogens from Wastewaters. Polymers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; De Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W. pH-responsive chitosan-based film incorporated with alizarin for intelligent packaging applications. Food Hydrocoll. 2020, 102, 105629. [Google Scholar] [CrossRef]
- Wang, F.-X.; Prokes, I.; Song, L.; Shi, H.; Sadler, P.J. Reactions of cisplatin and oxaliplatin with penicillin G: Implications for drug inactivation and biological activity. J. Biol. Inorg. Chem. 2022, 27, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Yang, X.; Wang, J.; Li, Y.; Yu, H.; Zhang, Y.; Liu, G. Advances in characterisation and biological activities of chitosan and chitosan oligosaccharides. Food Chem. 2016, 190, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Nourmoradi, H.; Daneshfar, A.; Mazloomi, S.; Bagheri, J.; Barati, S. Removal of Penicillin G from aqueous solutions by a cationic surfactant modified montmorillonite. MethodsX 2019, 6, 1967–1973. [Google Scholar] [CrossRef]
- Vakili, M.; Qiu, W.; Cagnetta, G.; Huang, J.; Yu, G. Mechanochemically oxidized chitosan-based adsorbents with outstanding Penicillin G adsorption capacity. J. Environ. Chem. Eng. 2021, 9, 105454. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Plazinski, W.; Dziuba, J.; Rudzinski, W. Modeling of sorption kinetics: The pseudo-second order equation and the sorbate intraparticle diffusivity. Adsorption 2013, 19, 1055–1064. [Google Scholar] [CrossRef]
- Ruderman, G.; Caffarena, E.; Mogilner, I.G.; Tolosa, E. José. Hydrogen Bonding of Carboxylic Acids in Aqueous Solutions—UV Spectroscopy, Viscosity, and Molecular Simulation of Acetic Acid. J. Solut. Chem. 1998, 27, 935–948. [Google Scholar] [CrossRef]







| Samples | Surface Area BET (m2 g−1) | Pore Surface Area (BJH) (m2 g−1) | Pore Diameter for Desorption (BJH) (nm) | Pore Volume (BJH) (Measured la P/P0 = 0.99) (cm3 g−1) |
|---|---|---|---|---|
| K-MAPTES | 7.373 | 9.216 | 28.750 | 0.034 |
| K | 9.703 | 10.010 | 3.169 | 0.024 |
| P1-K | 1.812 | 3.253 | 3.627 | 0.005 |
| P1-K-BC | 2.310 | 4.279 | 4.152 | 0.006 |
| P2-K | 3.705 | 6.177 | 4.152 | 0.010 |
| P3-K | 3.067 | 11.400 | 4.152 | 0.013 |
| P4-K | 1.318 | 2.273 | 4.752 | 0.004 |
| P4-K-BC | 2.213 | 3.068 | 4.543 | 0.005 |
| P5-K | 2.851 | 3.212 | 4.543 | 0.007 |
| P6-K | 3.980 | 5.053 | 4.543 | 0.010 |
| P7-K | 1.720 | 2.310 | 3.315 | 0.005 |
| P7-K-BC | 1.967 | 5.068 | 3.627 | 0.008 |
| P8-K | 6.484 | 9.480 | 3.969 | 0.018 |
| P9-K | 8.322 | 11.300 | 4.152 | 0.023 |
| Sample | k2 (g mg−1 min−1) | qe (mg/g) * | R2 |
|---|---|---|---|
| P1-K-BC | 5.37 × 10−6 | 7537 | 0.9999 |
| P1-K | 1.23 × 10−5 | 4491 | 0.9999 |
| P2-K | 1.17 × 10−5 | 4423 | 0.9999 |
| P3-K | 9.31 × 10−6 | 5918 | 0.9999 |
| P4-K-BC | 2.04 × 10−6 | 5742 | 0.9985 |
| P4-K | 5.34 × 10−6 | 3445 | 0.9998 |
| P5-K | 3.99 × 10−6 | 4247 | 0.9996 |
| P6-K | 2.66 × 10−5 | 2669 | 0.9995 |
| P7-K-BC | 3.83 × 10−6 | 6077 | 0.9995 |
| P7-K | 5.77 × 10−6 | 13,362 | 0.9999 |
| P8-K | 2.93 × 10−6 | 6378 | 0.9996 |
| P9-K | 2.98 × 10−6 | 4915 | 0.9993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitru, M.V.; Sandu, T.; Miron, A.; Zaharia, A.; Radu, I.C.; Gavrilă, A.-M.; Sârbu, A.; Iovu, H.; Chiriac, A.-L.; Iordache, T.V. Hybrid Cryogels with Superabsorbent Properties as Promising Materials for Penicillin G Retention. Gels 2023, 9, 443. https://doi.org/10.3390/gels9060443
Dumitru MV, Sandu T, Miron A, Zaharia A, Radu IC, Gavrilă A-M, Sârbu A, Iovu H, Chiriac A-L, Iordache TV. Hybrid Cryogels with Superabsorbent Properties as Promising Materials for Penicillin G Retention. Gels. 2023; 9(6):443. https://doi.org/10.3390/gels9060443
Chicago/Turabian StyleDumitru, Marinela Victoria, Teodor Sandu, Andreea Miron, Anamaria Zaharia, Ionuț Cristian Radu, Ana-Mihaela Gavrilă, Andrei Sârbu, Horia Iovu, Anita-Laura Chiriac, and Tanța Verona Iordache. 2023. "Hybrid Cryogels with Superabsorbent Properties as Promising Materials for Penicillin G Retention" Gels 9, no. 6: 443. https://doi.org/10.3390/gels9060443
APA StyleDumitru, M. V., Sandu, T., Miron, A., Zaharia, A., Radu, I. C., Gavrilă, A.-M., Sârbu, A., Iovu, H., Chiriac, A.-L., & Iordache, T. V. (2023). Hybrid Cryogels with Superabsorbent Properties as Promising Materials for Penicillin G Retention. Gels, 9(6), 443. https://doi.org/10.3390/gels9060443