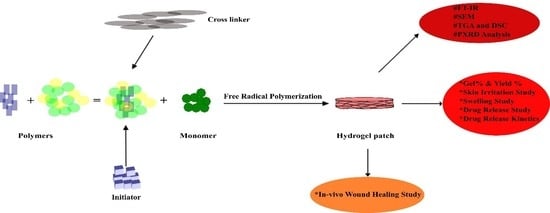
Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Macro-Morphological and Micro-Morphological Evaluation of Topical Hydrogel Patch
2.2. Physical Characteristics of Topical Hydrogel Patches
2.3. FTIR Analysis of Topical Hydrogel Patch
2.4. Differential Scanning Calorimetry (DSC) and Thermograviometric Analysis (TGA)
XRD Analysis of Topical Hydrogel Patch
2.5. Dynamic Swelling Studies of Topical Hydrogel Patch
2.6. Sol–Gel Analysis
2.7. In Vitro Drug Release Evaluation
2.8. Kinetic Modeling for Drug Release Profile
2.9. Ex Vivo Skin Drug Deposition Studies
2.10. Primary Skin Irritation Test
2.11. In Vivo Wound-Healing Potential
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation Method of Topical Hydrogel Patch
4.2.2. Macro-Morphological and Micro-Morphological Evaluation of Topical Hydrogel Patch
4.2.3. Physical Characteristics of Topical Hydrogel Patches
4.2.4. Characterization of Topical Hydrogel Patch for Polymer Compatibility and Thermal Stability
4.2.5. Sol–Gel Fraction
4.2.6. Dynamic Swelling Studies of Topical Patch
4.2.7. Neomycin-Loaded Topical Hydrogel Patch
4.2.8. In Vitro Drug Release Evaluation
4.2.9. Release Kinetic Study of Ivabradine HCl
4.2.10. Ex Vivo Skin Deposition Analysis
4.2.11. Primary Skin Irritation Test
4.2.12. In Vivo Wound-Healing Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ali, S.; Shabbir, M.; Shahid, N. The structure of skin and transdermal drug delivery system—A review. Res. J. Pharm. Technol. 2015, 8, 103–109. [Google Scholar] [CrossRef]
- Shabbir, M.; Nagra, U.; Zaman, M.; Mahmood, A.; Barkat, K. Lipid vesicles and nanoparticles for non-invasive topical and transdermal drug delivery. Curr. Pharm. Des. 2020, 26, 2149–2166. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Amano, S. Increment of subcutaneous adipose tissue is associated with decrease of elastic fibres in the dermal layer. Exp. Dermatol. 2015, 24, 924–929. [Google Scholar] [CrossRef]
- Whitney, J.D. Overview: Acute and chronic wounds. Nurs. Clin. 2005, 40, 191–205. [Google Scholar] [CrossRef]
- Tavakoli, S.; Klar, A.S. Advanced hydrogels as wound dressings. Biomolecules 2020, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, H.; Pan, X.; Zhang, C.; Zhang, K.; Chen, Z.; Dong, W.; Xie, A.; Qi, X. Dendritic Hydrogels with Robust Inherent Antibacterial Properties for Promoting Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 11144–11155. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Knill, C.J.; Thorley, M. Natural polymers for healing wounds. In Recent Advances in Environmentally Compatible Polymers; Elsevier: Amsterdam, The Netherlands, 2001; pp. 97–104. [Google Scholar]
- Kamoun, E.A.; Chen, X.; Eldin, M.S.M.; Kenawy, E.-R.S. Crosslinked poly (vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Swamy, B.Y.; Chang, J.H.; Ahn, H.; Lee, W.-K.; Chung, I. Thermoresponsive N-vinyl caprolactam grafted sodium alginate hydrogel beads for the controlled release of an anticancer drug. Cellulose 2013, 20, 1261–1273. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, F. Synthesis and properties of temperature-sensitive hydrogel based on hydroxyethyl cellulose. Int. J. Polym. Mater. 2010, 59, 450–461. [Google Scholar] [CrossRef]
- Ebnesajjad, S. 8—Characteristics of Adhesive Materials. In Handbook of Adhesives and Surface Preparation; Ebnesajjad, S., Ed.; William Andrew Publishing: Oxford, UK, 2011; pp. 137–183. [Google Scholar] [CrossRef]
- Macdonald, R.; Beck, M. Neomycin: A review with particular reference to dermatological usage. Clin. Exp. Dermatol. 1983, 8, 249–258. [Google Scholar] [CrossRef]
- Rogers, C.M.; Scott, E.M.; Sarawichitr, B.; Arnold, C.; Suchodolski, J.S. Evaluation of the bacterial ocular surface microbiome in ophthalmologically normal dogs prior to and following treatment with topical neomycin-polymyxin-bacitracin. PLoS ONE 2020, 15, e0234313. [Google Scholar] [CrossRef]
- Nagra, U.; Barkat, K.; Ashraf, M.U.; Shabbir, M. Feasibility of Enhancing Skin Permeability of Acyclovir through Sterile Topical Lyophilized Wafer on Self-Dissolving Microneedle-Treated Skin. Dose Response 2022, 20, 15593258221097594. [Google Scholar] [CrossRef]
- Shabbir, M.; Sajid, A.; Hamid, I.; Sharif, A.; Akhtar, M.F.; Raza, M.; Ahmed, S.; Peerzada, S.; Amin, M.U. Influence of different formulation variables on the performance of transdermal drug delivery system containing tizanidine hydrochloride: In vitro and ex vivo evaluations. Braz. J. Pharm. Sci. 2019, 54, e00130. [Google Scholar] [CrossRef] [Green Version]
- Arshad, I.; Ali, S.; Amin, U.; Shabbir, M.; Raza, M.; Sharif, A.; Akhtar, M.F. Effect of hydrophilic and hydrophobic polymer on the release of ketoprofen and allopurinol from bilayer matrix transdermal patch. Adv. Polym. Technol. 2018, 37, 3076–3083. [Google Scholar] [CrossRef]
- Rastogi, L.; Kora, A.J.; Arunachalam, J. Highly stable, protein capped gold nanoparticles as effective drug delivery vehicles for amino-glycosidic antibiotics. Mater. Sci. Eng. C 2012, 32, 1571–1577. [Google Scholar] [CrossRef]
- El Barkany, S.; El Idrissi, A.; Ouslimane, S.; Amhamdi, H. Optimization of the experimental conditions for the extraction of cellulose starting from the “stipa tenacissima” of Eastern Morocco. Phys. Chem. News 2009, 46, 135–141. [Google Scholar]
- Pastorova, I.; Botto, R.E.; Arisz, P.W.; Boon, J.J. Cellulose char structure: A combined analytical Py-GC-MS, FTIR, and NMR study. Carbohydr. Res. 1994, 262, 27–47. [Google Scholar] [CrossRef]
- Aprilliza, M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf. Series Mater. Sci. Eng. 2017, 188, 012019. [Google Scholar]
- Reddy, B.V.; Rao, G.R. Vibrational spectra and modified valence force field for N, N-methylenebisacrylamide. Indian J. Pure Appl. Phys. 2008, 46, 611–616. [Google Scholar]
- Çaykara, T.; Demirci, S. Preparation and characterization of blend films of poly (vinyl alcohol) and sodium alginate. J. Macromol. Sci. Part A 2006, 43, 1113–1121. [Google Scholar] [CrossRef]
- Azzaouia, K.; Mejdoubia, E.; Lamhamdia, A.; Lakrata, M.; Hamedb, O.; Jodehb, S.; Berrabaha, M.; Elidrissic, A.; El Meskinid, I.; Daoudid, M. Preparation of hydroxyapatite biobased microcomposite film for selective removal of toxic dyes from wastewater. Polymer 2019, 1, 28. [Google Scholar]
- Soares, J.d.P.; Santos, J.; Chierice, G.O.; Cavalheiro, E. Thermal behavior of alginic acid and its sodium salt. Eclética Química 2004, 29, 57–64. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, N.S.; Awad, H.; El-Sayed, G.M.; Nagieb, Z.A.; Kamel, S. Synthesis and characterization of biocompatible hydrogel based on hydroxyethyl cellulose-g-poly (hydroxyethyl methacrylate). Polym. Bull. 2020, 77, 6333–6347. [Google Scholar] [CrossRef]
- Sand, A.; Yadav, M.; Mishra, D.K.; Behari, K. Modification of alginate by grafting of N-vinyl-2-pyrrolidone and studies of physicochemical properties in terms of swelling capacity, metal-ion uptake and flocculation. Carbohydr. Polym. 2010, 80, 1147–1154. [Google Scholar] [CrossRef]
- Jana, S.; Trivedi, M.K.; Tallapragada, R.M.; Branton, A.; Trivedi, D.; Nayak, G.; Mishra, R. Characterization of physicochemical and thermal properties of chitosan and sodium alginate after biofield treatment. Pharm. Anal. Acta 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.; Nayak, G.; Patil, S.; Tallapragada, R.M.; Mishra, R. Influence of biofield treatment on physicochemical properties of hydroxyethyl cellulose and hydroxypropyl cellulose. J. Mol. Pharm. Org. Process Res. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Wang, A. Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly (sodium acrylate) and polyvinylpyrrolidone. Carbohydr. Polym. 2010, 80, 1028–1036. [Google Scholar] [CrossRef]
- Kajjari, P.B.; Manjeshwar, L.S.; Aminabhavi, T.M. Semi-interpenetrating polymer network hydrogel blend microspheres of gelatin and hydroxyethyl cellulose for controlled release of theophylline. Ind. Eng. Chem. Res. 2011, 50, 7833–7840. [Google Scholar] [CrossRef]
- Manjula, B.; Varaprasad, K.; Sadiku, R.; Raju, K.M. Preparation and characterization of sodium alginate–based hydrogels and their in vitro release studies. Adv. Polym. Technol. 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly (acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Sullad, A.G.; Manjeshwar, L.S.; Aminabhavi, T.M. Novel pH-sensitive hydrogels prepared from the blends of poly (vinyl alcohol) with acrylic acid-graft-guar gum matrixes for isoniazid delivery. Ind. Eng. Chem. Res. 2010, 49, 7323–7329. [Google Scholar] [CrossRef]
- Peng, G.; Xu, S.; Peng, Y.; Wang, J.; Zheng, L. A new amphoteric superabsorbent hydrogel based on sodium starch sulfate. Bioresour. Technol. 2008, 99, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Naeem, S.; Barkat, K.; Shabbir, M.; Khalid, I.; Anjum, I.; Shamshad, N.; Mehmood, Y.; Khan, D.H.; Badshah, S.F.; Syed, M.A. Fabrication of pH responsive hydrogel blends of chondroitin sulfate/pluronic F-127 for the controlled release of ketorolac: Its characterization and acute oral toxicity study. Drug Dev. Ind. Pharm. 2022, 48, 611–622. [Google Scholar] [CrossRef]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly (acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Shabbir, M.; Barkat, K.; Ashraf, M.U.; Nagra, U. Development of a Novel Self-Dissolving Microneedle-Assisted Percutaneous Delivery System of Diacerein through Solid Dispersion Gel: Solubility Enhancement, Proof of Anti-inflammatory Activity and Safety. Curr. Drug Deliv. 2023, 20, 1351–1367. [Google Scholar]
- Shabbir, M.; Ali, S.; Farooq, M.; Adnan, S.; Yousaf, M.; Idrees, A.; Rehman, K.; Shahid, N. Formulation factors affecting in vitro and ex vivo permeation of bisoprolol fumarate from a matrix transdermal patch. Adv. Polym. Technol. 2016, 35, 237–247. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Yuan, Z.; Han, H.; Li, T.; Li, L.; Guo, X. Chitosan cross-linked poly (acrylic acid) hydrogels: Drug release control and mechanism. Colloids Surf. B Biointerfaces 2017, 152, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Minhas, M.U.; Ahmad, M.; Sohail, M.; Abdullah, O.; Badshah, S.F. Preparation and evaluation of skin wound healing chitosan-based hydrogel membranes. AAPS PharmSciTech 2018, 19, 3199–3209. [Google Scholar] [CrossRef]
- Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S.; Han, S.S. Gentamicin-loaded wound dressing with polyvinyl alcohol/dextran hydrogel: Gel characterization and in vivo healing evaluation. AAPS PharmSciTech 2010, 11, 1092–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, B.J.; Kim, A.; Park, S.N. Properties and in vitro drug release of hyaluronic acid-hydroxyethyl cellulose hydrogels for transdermal delivery of isoliquiritigenin. Carbohydr. Polym. 2016, 147, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, M.; Ali, S.; Raza, M.; Sharif, A.; Akhtar, F.M.; Manan, A.; Fazli, A.R.; Younas, N.; Manzoor, I. Effect of hydrophilic and hydrophobic polymer on in vitro dissolution and permeation of bisoprolol fumarate through transdermal patch. Acta Pol. Pharm. 2017, 74, 187–197. [Google Scholar]
- Suhag, G.S.; Bhatnagar, A.; Singh, H. Poly (hydroxyethyl methacrylate)-based co-polymeric hydrogels for transdermal delivery of salbutamol sulphate. J. Biomater. Sci. Polym. Ed. 2008, 19, 1189–1200. [Google Scholar] [CrossRef] [PubMed]








| Formulation | Thickness (mm) | Weight Variation (g) | Folding Endurance |
|---|---|---|---|
| HECA-1 | 1.75 ± 0.05 | 2.41 ± 0.30 | 333 ± 20 |
| HECA-2 | 1.78 ± 0.13 | 2.43 ± 0.21 | 342 ± 15 |
| HECA-3 | 1.80 ± 0.07 | 2.47 ± 0.16 | 348 ± 08 |
| HECA-4 | 1.82 ± 0.18 | 2.49 ± 0.24 | 350 ± 12 |
| HECA-5 | 1.86 ± 0.11 | 2.65 ± 0.09 | 356 ± 16 |
| HECA-6 | 1.89 ± 0.09 | 2.76 ± 0.15 | 362 ± 23 |
| HECA-7 | 1.83 ± 0.16 | 2.50 ± 0.10 | 355 ± 21 |
| HECA-8 | 1.91 ± 0.05 | 2.81 ± 0.07 | 368 ± 12 |
| HECA-9 | 1.95 ± 0.08 | 2.94 ± 0.16 | 374 ± 09 |
| HECA-10 | 1.81 ± 0.11 | 2.48 ± 0.13 | 335 ± 16 |
| HECA-11 | 1.85 ± 0.06 | 2.57 ± 0.18 | 315 ± 18 |
| HECA-12 | 1.87 ± 0.02 | 2.62 ± 0.12 | 320 ± 10 |
| Formulations | Zero-Order | First-Order | Higuchi Model | Korsmeyer–Peppas | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| HECA-1 | 0.9865 | 0.6104 | 0.9627 | 0.9246 | 0.521 |
| HECA-2 | 0.9801 | 0.5299 | 0.9475 | 0.8968 | 0.496 |
| HECA-3 | 0.9854 | 0.5869 | 0.9644 | 0.9296 | 0.509 |
| HECA-4 | 0.9858 | 0.5872 | 0.9646 | 0.9299 | 0.505 |
| HECA-5 | 0.9896 | 0.2936 | 0.9804 | 0.9626 | 0.415 |
| HECA-6 | 0.9893 | 0.1618 | 0.9735 | 0.9553 | 0.455 |
| HECA-7 | 0.9855 | 0.5862 | 0.9645 | 0.9293 | 0.499 |
| HECA-8 | 0.9952 | 0.4239 | 0.9847 | 0.9719 | 0.444 |
| HECA-9 | 0.9934 | 0.3191 | 0.9848 | 0.9712 | 0.419 |
| HECA-10 | 0.9862 | 0.5867 | 0.9644 | 0.9295 | 0.504 |
| HECA-11 | 0.9929 | 0.5573 | 0.9762 | 0.9532 | 0.489 |
| HECA-12 | 0.9949 | 0.6062 | 0.9856 | 0.9714 | 0.501 |
| Formulation | SA (g) | HEC (g) | AA (g) | APS (g) | MBA (g) |
|---|---|---|---|---|---|
| HECA-1 | 0.1 | 0.01 | 4 | 0.1 | 0.1 |
| HECA-2 | 0.1 | 0.02 | 4 | 0.1 | 0.1 |
| HECA-3 | 0.1 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-4 | 0.1 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-5 | 0.15 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-6 | 0.2 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-7 | 0.1 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-8 | 0.1 | 0.03 | 5 | 0.1 | 0.1 |
| HECA-9 | 0.1 | 0.03 | 6 | 0.1 | 0.1 |
| HECA-10 | 0.1 | 0.03 | 4 | 0.1 | 0.1 |
| HECA-11 | 0.1 | 0.03 | 4 | 0.1 | 0.15 |
| HECA-12 | 0.1 | 0.03 | 4 | 0.1 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, S.; Barkat, K.; Ashraf, M.U.; Shabbir, M.; Anjum, I.; Badshah, S.F.; Aamir, M.; Malik, N.S.; Tariq, A.; Ullah, R. Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing. Gels 2023, 9, 567. https://doi.org/10.3390/gels9070567
Saeed S, Barkat K, Ashraf MU, Shabbir M, Anjum I, Badshah SF, Aamir M, Malik NS, Tariq A, Ullah R. Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing. Gels. 2023; 9(7):567. https://doi.org/10.3390/gels9070567
Chicago/Turabian StyleSaeed, Sana, Kashif Barkat, Muhammad Umer Ashraf, Maryam Shabbir, Irfan Anjum, Syed Faisal Badshah, Muhammad Aamir, Nadia Shamshad Malik, Akash Tariq, and Riaz Ullah. 2023. "Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing" Gels 9, no. 7: 567. https://doi.org/10.3390/gels9070567
APA StyleSaeed, S., Barkat, K., Ashraf, M. U., Shabbir, M., Anjum, I., Badshah, S. F., Aamir, M., Malik, N. S., Tariq, A., & Ullah, R. (2023). Flexible Topical Hydrogel Patch Loaded with Antimicrobial Drug for Accelerated Wound Healing. Gels, 9(7), 567. https://doi.org/10.3390/gels9070567