Cellulose Nanocrystal (CNC) Gels: A Review
Abstract
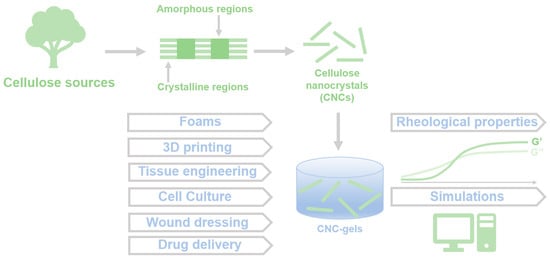
:1. Introduction
2. Preparation and Properties of CNC Gels
2.1. Preparation Methods
2.1.1. Preparation of CNC-Based Hydrogels
2.1.2. Hydrogels with Physically Cross-Linked CNCs
2.1.3. Hydrogels with Chemically Cross-Linked CNCs
2.2. Rheological Properties
| Reference | Nanocomposites Hydrogel | Production Methods | Aim | Type of Rheology Characterization | Application |
|---|---|---|---|---|---|
| Tang et al. (2020) [25] | Oxidized cellulose nanocrystal (CNC-CHO) and oxidized sodium alginate (Alg-CHO) | Alg-CHO and various amounts ofCNC-CHO were mixed with water. Polyacrylamide was used as macro-cross-linkers. | The aim was to study the oxidized alginate-polyacrylamide hydrogels reinforced with different amounts of CNC Oxidized (CNC-CHO) (0.5, 1.0, 1.5 wt%) on hydrogels’ properties. | The storage modulus, loss modulus and complex viscosity were measured a as function of frequency. In addition, the elastic modulus was also evaluated as a function of CNC-CHO concentration. | Agricultural and pharmaceuticalsectors |
| Talantikite et al. (2019) [64] | Cellulose nanocrystals (CNC) and xyloglucan (XG) | Dispersion of CNC into XG solution. | The aim was to study the effect of XG molar mass (100, 300 and 800) on mechanical properties containing a constant amount of CNC. | The effect of XG molar mass on the elastic (G’) and viscous (G”) modulus were evaluated a as function of frequency. | Biomedical field, cosmetic, chromatography, food and also in domestic uses. |
| Liu et al. (2018) [102] | Cellulose nanocrystals pure and modified (CNC-NH2) and Polysaccharide mixture (Cellulose acetoacetate (CAA) and Hydroxyproyl chitosan (HPCS)) | Dispersion of CNCs pure and CNCs modified into polysaccharide mixture solution. | The aim was to study the effect of different concentrations of CNCs pure and CNCs modified (CNC-NH2) (0.2, 0.4, 0.6, 0.8 and 1 wt%) on polysaccharide hydrogels properties (CAA/HPCS). | The storage modulus (G’) was measured as a function of different loading concentration of CNCs pure and CNCs modified (CNC-NH2) into CAA/HPCS hydrogel. | Biomedical field |
| Hou et al. (2017) [105] | Cellulose nanocrystals (CNCs) and Poly(ethylene glycol) diacrylate (PEGDA) oligomer | Dispersion of CNCs pure into Poly(ethylene glycol) diacrylate (PEGDA). | They studied the effect of hydrogen bonds and different concentrations of CNC on viscosity of CNC/PEGDA mixture to produce hydrogel filament using DCS (dynamic-cross-linking-spinning) method. | The storage modulus (G’), loss modulus (G”) and loss factor (tanδ) were measured a as function of frequency. Additionally measured were shear stress and viscosity a as function of shear rate, and viscosity a as function of CNC content. | Biomedical applications |
| Ling Zhou et al. (2016) [106] | Cellulose nanofibers (CNFs), rod-like cellulosenanocrystals (CNCs) and spherical cellulose nanocrystals (SCNCs) and poly (vinyl alcohol) (PVA) | Dispersion of CNFs, CNCs and SCNCs into poly (vinyl alcohol) (PVA) suspension. | The aim of this work was to study the effect of morphology of different kinds of cellulose nanoparticles (CNs) and amount of CNs (3 wt%, 6 wt% or 9 wt%) into poly (vinyl alcohol) (PVA) on viscosity. | Steady-state viscosities as a function of the shear rate, as well as storage modulus (G’), loss modulus (G”), and loss tangent tan as a function of angular for various CN and PVA/CN suspensions were measured. | Fibers and films |
| Ooi et al. (2016) [76] | CNC and gelatin (Pharmaceutical grade—not specified the name) | Dispersion of gelatin into the CNC suspension. (Glutaraldehyde was added for cross-linking between gelatin chains). | The aim of this work was to study the effect of different content of CNC (5, 10, 15, 20 and 25%) on dynamic mechanical properties of the CNC/gelatin hydrogel. | The storage modulus (G’) and loss modulus (G’’) as a function of angular frequency were determined using different CNC concentrations into CNC/gelatin solutions. | Drug delivery system. |
| You et al. (2016) [16] | Cationic cellulose nanocrystals (CCNCs) and Quaternized cellulose (QC) and the β-glycerophosphate (β-GP) | QC was dissolved in water dispersion of CCNC with different content. (The β-glycerophosphate (β-GP) was added and used like a cross-linking agent for the interaction between CCNCs and QC chains). | The aim of this work was to study the effect of different content of CCNC (1, 1.5 and 2.5%) in CCNC suspensions and QC/CCNC/β-GP mixtures on dynamic mechanical properties of the hydrogel produced. | First the steady shear viscosity a as function of shear rate was determined in CCNC suspensions with various contents. After, the storage modulus (G’) and loss modulus (G’’) as a function of different temperatures and storage modulus (G’) as a function of angular frequency were determined using different CCNC concentrations in QC/ β-GP solutions or suspensions. | Biomedical application (for example injectable products). |
| Mihranyan (2013) [83] | Microcrystalline cellulose (MCC) whiskers with PVA | MCC was dissolved in water dispersion after PVA was added. TEMPO ((2,2,6,6-Tetramethylpiperidin-1-yl)oxyl) was used to modify the surface of MCC for cross-linking between MCC and PVA. | The aim of this work was to study the feasibility of direct chemical cross-linking of microcrystalline cellulose whiskers with PVA on mechanical properties of PVA hydrogels. | Frequency dependence of the elastic (storage) modulus G’, viscous (loss) modulus G’’ and damping factor tan δ of 3MCC–PVAhydrogels were measured. | Biomedical orthopedic application |
| Han et al. (2013) [47] | CNPs (CNCs and CNFs) and PVA without and with borax (chemical cross-linking agent for PVA) hydrogels). | Dispersion of CNFs and CNCs into poly (vinyl alcohol) (PVA) with borax. | The aim of this work was to study the influence of various cellulose nanocrystal particles (CNCs and CNFs) and borax on the viscoelastic properties of hydrogels. | The storage modulus (G’) (elasticity) and complex viscosity (η*) as a function of angular frequency were determined. In addition, the steadyshear viscosity (η) versus shear rate was also measured. | Biosensors, medical implants, and even drug-deliverydevices |
| Zhou et al. (2011) [79] | Cellulose nanocrystals (CNCs) and polyacrylamide (PAM) | Rod-shaped cellulose nanocrystals (CNCs) were added into polyacrylamide (PAM) and hydrogels were produced through in situ free-radical polymerization in the presence ofcross-linker N,N0-methylenebisacrylamide (NMBA). | The aim of this work was to study the influence of polymerization time and effect of CNC content on elastic modulus (G’). | The elastic modulus (G’) as a function of polymerization time for different CNC content was measured. In addition, the effect of CNC content on induction of gels was determined. | PAM hydrogels have a wide variety of applications in agriculture, drilling fluids, tissue engineering, and waste treatments. |
3. Simulation/Numerical Approach
4. Insights and Perspectives
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le Gars, M.; Douard, L.; Belgacem, N.; Bras, J. Cellulose Nanocrystals: From Classical Hydrolysis to the Use of Deep Eutectic Solvents. In Smart Nanosystems for Biomedicine, Optoelectronics and Catalysis; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Xie, H.; Du, H.; Yang, X.; Si, C. Recent Strategies in Preparation of Cellulose Nanocrystals and Cellulose Nanofibrils Derived from Raw Cellulose Materials. Int. J. Polym. Sci. 2018, 2018, 7923068. [Google Scholar] [CrossRef]
- Vanderfleet, O.M.; Cranston, E.D. Production Routes to Tailor the Performance of Cellulose Nanocrystals. Nat. Rev. Mater. 2020, 6, 124–144. [Google Scholar] [CrossRef]
- Capron, I.; Rojas, O.J.; Bordes, R. Behavior of Nanocelluloses at Interfaces. Curr. Opin. Colloid Interface Sci. 2017, 29, 83–95. [Google Scholar] [CrossRef]
- Miyashiro, D.; Hamano, R.; Umemura, K. A Review of Applications Using Mixed Materials of Cellulose, Nanocellulose and Carbon Nanotubes. Nanomaterials 2020, 10, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domingues, R.M.A.; Gomes, M.E.; Reis, R.L. The Potential of Cellulose Nanocrystals in Tissue Engineering Strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, L.; Li, M.-C.; Wu, Q.; Zhou, D. Cellulose Nanocrystals (CNCs) from Corn Stalk: Activation Energy Analysis. Materials 2017, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.; Qin, Z.; Chen, Y.; Hu, S.; Ren, Z.; Zhu, M. Efficient Extraction of Cellulose Nanocrystals through Hydrochloric Acid Hydrolysis Catalyzed by Inorganic Chlorides under Hydrothermal Conditions. ACS Sustain. Chem. Eng. 2017, 5, 4656–4664. [Google Scholar] [CrossRef]
- Cheng, M.; Qin, Z.; Hu, J.; Liu, Q.; Wei, T.; Li, W.; Ling, Y.; Liu, B. Facile and Rapid One–Step Extraction of Carboxylated Cellulose Nanocrystals by H2SO4/HNO3 Mixed Acid Hydrolysis. Carbohydr. Polym. 2020, 231, 115701. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.N.M.; Hosseinmardi, A.; Martin, D.J.; Annamalai, P.K. A Mixed Acid Methodology to Produce Thermally Stable Cellulose Nanocrystal at High Yield Using Phosphoric Acid. J. Bioresour. Bioprod. 2022, 7, 99–108. [Google Scholar] [CrossRef]
- Sofla, M.R.K.; Brown, R.J.; Tsuzuki, T.; Rainey, T.J. A Comparison of Cellulose Nanocrystals and Cellulose Nanofibres Extracted from Bagasse Using Acid and Ball Milling Methods. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 035004. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Annamalai, P.K.; Morrow, I.C.; Martin, D. Production of Cellulose Nanocrystals via a Scalable Mechanical Method. RSC Adv. 2015, 5, 57133–57140. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Hamad, W.Y. Cellulose Nanocrystals: Properties, Production and Applications; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-96816-0. [Google Scholar]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Cao, J.; Zhao, Y.; Zhang, L.; Zhou, J.; Chen, Y. Improved Mechanical Properties and Sustained Release Behavior of Cationic Cellulose Nanocrystals Reinforeced Cationic Cellulose Injectable Hydrogels. Biomacromolecules 2016, 17, 2839–2848. [Google Scholar] [CrossRef] [PubMed]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose Nanocrystal Based Composites: A Review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current International Research into Cellulose Nanofibres and Nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose Nanocrystals and Cellulose Nanofibrils Based Hydrogels for Biomedical Applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef] [Green Version]
- Shojaeiarani, J.; Bajwa, D.; Shirzadifar, A. A Review on Cellulose Nanocrystals as Promising Biocompounds for the Synthesis of Nanocomposite Hydrogels. Carbohydr. Polym. 2019, 216, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Eom, Y. Review on Cellulose Nanocrystal-Reinforced Polymer Nanocomposites: Processing, Properties, and Rheology. Korea-Aust. Rheol. J. 2021, 33, 165–185. [Google Scholar] [CrossRef]
- Tang, J.; Javaid, M.U.; Pan, C.; Yu, G.; Berry, R.M.; Tam, K.C. Self-Healing Stimuli-Responsive Cellulose Nanocrystal Hydrogels. Carbohydr. Polym. 2020, 229, 115486. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Chau, M.; Sriskandha, S.E.; Pichugin, D.; Thérien-Aubin, H.; Nykypanchuk, D.; Chauve, G.; Méthot, M.; Bouchard, J.; Gang, O.; Kumacheva, E. Ion-Mediated Gelation of Aqueous Suspensions of Cellulose Nanocrystals. Biomacromolecules 2015, 16, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Way, A.E.; Hsu, L.; Shanmuganathan, K.; Weder, C.; Rowan, S.J. PH-Responsive Cellulose Nanocrystal Gels and Nanocomposites. ACS Macro Lett. 2012, 1, 1001–1006. [Google Scholar] [CrossRef]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological Behavior and Particle Alignment of Cellulose Nanocrystal and Its Composite Hydrogels during 3D Printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef] [PubMed]
- Trache, D. Nanocellulose as a Promising Sustainable Material for Biomedical Applications. AIMS Mater. Sci. 2018, 5, 201–205. [Google Scholar] [CrossRef]
- Sultan, S.; Siqueira, G.; Zimmermann, T.; Mathew, A.P. 3D Printing of Nano-Cellulosic Biomaterials for Medical Applications. Curr. Opin. Biomed. Eng. 2017, 2, 29–34. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.Y.; Wang, Y.; Jin, Y.; Guo, X.; Liu, Y.Y.; Qi, X.; Lei, H.; Xu, H. Heat-Induced Whey Protein Isolate Gels Improved by Cellulose Nanocrystals: Gelling Properties and Microstructure. Carbohydr. Polym. 2020, 231, 115749. [Google Scholar] [CrossRef]
- Li, M.; Wu, Q.; Moon, R.J.; Hubbe, M.A.; Bortner, M.J. Rheological Aspects of Cellulose Nanomaterials: Governing Factors and Emerging Applications. Adv. Mater. 2021, 33, 2006052. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Sherje, A.P. Cellulose Nanocrystals: Fundamentals and Biomedical Applications. Carbohydr. Polym. 2022, 275, 118668. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Fan, H.; Zhang, X.; Haq, F.; Ullah, A.; Ullah, R.; Khan, F.U.; Iqbal, M. Advance Study of Cellulose Nanocrystals Properties and Applications. J. Polym. Environ. 2020, 28, 1117–1128. [Google Scholar] [CrossRef]
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose Nanocrystals: Pretreatments, Preparation Strategies, and Surface Functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef] [Green Version]
- Boluk, Y.; Zhao, L.; Incani, V. Dispersions of Nanocrystalline Cellulose in Aqueous Polymer Solutions: Structure Formation of Colloidal Rods. Langmuir 2012, 28, 6114–6123. [Google Scholar] [CrossRef]
- Hu, Z.; Cranston, E.D.; Ng, R.; Pelton, R. Tuning Cellulose Nanocrystal Gelation with Polysaccharides and Surfactants. Langmuir 2014, 30, 2684–2692. [Google Scholar] [CrossRef]
- Lewis, L.; Derakhshandeh, M.; Hatzikiriakos, S.G.; Hamad, W.Y.; MacLachlan, M.J. Hydrothermal Gelation of Aqueous Cellulose Nanocrystal Suspensions. Biomacromolecules 2016, 17, 2747–2754. [Google Scholar] [CrossRef]
- Shafeiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Influence of Degree of Sulfation on the Rheology of Cellulose Nanocrystal Suspensions. Rheol. Acta 2013, 52, 741–751. [Google Scholar] [CrossRef]
- Shafiei-Sabet, S.; Hamad, W.Y.; Hatzikiriakos, S.G. Rheology of Nanocrystalline Cellulose Aqueous Suspensions. Langmuir 2012, 28, 17124–17133. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Kitchens, C.L. Wide-Angle X-ray Diffraction of Cellulose Nanocrystal−Alginate Nanocomposite Fibers. Macromolecules 2011, 44, 3478–3484. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Dorris, A.; Gray, D.G. Gelation of Cellulose Nanocrystal Suspensions in Glycerol. Cellulose 2012, 19, 687–694. [Google Scholar] [CrossRef]
- Moroni, L.; Burdick, J.A.; Highley, C.; Lee, S.J.; Morimoto, Y.; Takeuchi, S.; Yoo, J.J. Biofabrication Strategies for 3D in Vitro Models and Regenerative Medicine. Nat. Rev. Mater. 2018, 3, 21–37. [Google Scholar] [CrossRef]
- Han, J.; Lei, T.; Wu, Q. Facile Preparation of Mouldable Polyvinyl Alcohol-Borax Hydrogels Reinforced by Well-Dispersed Cellulose Nanoparticles: Physical, Viscoelastic and Mechanical Properties. Cellulose 2013, 20, 2947–2958. [Google Scholar] [CrossRef]
- McKee, J.R.; Appel, E.A.; Seitsonen, J.; Kontturi, E.; Scherman, O.A.; Ikkala, O. Healable, Stable and Stiff Hydrogels: Combining Conflicting Properties Using Dynamic and Selective Three-Component Recognition with Reinforcing Cellulose Nanorods. Adv. Funct. Mater. 2014, 24, 2706–2713. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption Kinetic and Equilibrium Studies for Methylene Blue Dye by Partially Hydrolyzed Polyacrylamide/Cellulose Nanocrystal Nanocomposite Hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Lin, N.; Gèze, A.; Wouessidjewe, D.; Huang, J.; Dufresne, A. Biocompatible Double-Membrane Hydrogels from Cationic Cellulose Nanocrystals and Anionic Alginate as Complexing Drugs Codelivery. ACS Appl. Mater. Interfaces 2016, 8, 6880–6889. [Google Scholar] [CrossRef]
- Wang, K.; Nune, K.C.; Misra, R.D.K. The Functional Response of Alginate-Gelatin-Nanocrystalline Cellulose Injectable Hydrogels toward Delivery of Cells and Bioactive Molecules. Acta Biomater. 2016, 36, 143–151. [Google Scholar] [CrossRef]
- Le Goff, K.J.; Gaillard, C.; Helbert, W.; Garnier, C.; Aubry, T. Rheological Study of Reinforcement of Agarose Hydrogels by Cellulose Nanowhiskers. Carbohydr. Polym. 2015, 116, 117–123. [Google Scholar] [CrossRef]
- Sanna, R.; Fortunati, E.; Alzari, V.; Nuvoli, D.; Terenzi, A.; Casula, M.F.; Kenny, J.M.; Mariani, A. Poly(N-Vinylcaprolactam) Nanocomposites Containing Nanocrystalline Cellulose: A Green Approach to Thermoresponsive Hydrogels. Cellulose 2013, 20, 2393–2402. [Google Scholar] [CrossRef]
- Gonzalez, J.S.; Ludueña, L.N.; Ponce, A.; Alvarez, V.A. Poly(Vinyl Alcohol)/Cellulose Nanowhiskers Nanocomposite Hydrogels for Potential Wound Dressings. Mater. Sci. Eng. C 2014, 34, 54–61. [Google Scholar] [CrossRef]
- Abitbol, T.; Johnstone, T.; Quinn, T.M.; Gray, D.G. Reinforcement with Cellulose Nanocrystals of Poly(Vinyl Alcohol) Hydrogels Prepared by Cyclic Freezing and Thawing. Soft Matter 2011, 7, 2373. [Google Scholar] [CrossRef]
- Aouada, F.A.; de Moura, M.R.; Orts, W.J.; Mattoso, L.H.C. Preparation and Characterization of Novel Micro- and Nanocomposite Hydrogels Containing Cellulosic Fibrils. J. Agric. Food Chem. 2011, 59, 9433–9442. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, S.K.; Pathak, V.; Soni, B.; Mohan, Y.M. CNWs Loaded Poly(SA) Hydrogels: Effect of High Concentration of CNWs on Water Uptake and Mechanical Properties. Carbohydr. Polym. 2014, 106, 351–358. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Pathak, V.; Chand, N.; Soni, B. Cellulose Nano Whiskers (CNWs) Loaded-Poly(Sodium Acrylate) Hydrogels. Part-I. Effect of Low Concentration of CNWs on Water Uptake. J. Macromol. Sci. Part A 2013, 50, 466–477. [Google Scholar] [CrossRef]
- Karaaslan, M.A.; Tshabalala, M.A.; Yelle, D.J.; Buschle-Diller, G. Nanoreinforced Biocompatible Hydrogels from Wood Hemicelluloses and Cellulose Whiskers. Carbohydr. Polym. 2011, 86, 192–201. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.A.; Pereira, A.G.B.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent Hydrogel Composite Made of Cellulose Nanofibrils and Chitosan-Graft-Poly(Acrylic Acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive Photonic Hydrogels Based on Nanocrystalline Cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Mechanical and Viscoelastic Properties of Cellulose Nanocrystals Reinforced Poly(Ethylene Glycol) Nanocomposite Hydrogels. ACS Appl. Mater. Interfaces 2013, 5, 3199–3207. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.-M.; Xu, F. Design of Cellulose Nanocrystals Template-Assisted Composite Hydrogels: Insights from Static to Dynamic Alignment. Macromolecules 2015, 48, 1231–1239. [Google Scholar] [CrossRef]
- Talantikite, M.; Gourlay, A.; Le Gall, S.; Cathala, B. Influence of Xyloglucan Molar Mass on Rheological Properties of Cellulose Nanocrystal/Xyloglucan Hydrogels. J. Renew. Mater. 2019, 7, 1381–1390. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yang, Y.; Peng, H.; Whittaker, A.K.; Li, Y.; Zhao, Q.; Wang, Y.; Zhu, S.; Wang, Z. Cellulose Nanocrystals Reinforced Highly Stretchable Thermal-Sensitive Hydrogel with Ultra-High Drug Loading. Carbohydr. Polym. 2021, 266, 118122. [Google Scholar] [CrossRef]
- Aswathy, S.H.; Narendrakumar, U.; Manjubala, I. Commercial Hydrogels for Biomedical Applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- You, C.; Ning, L.; Wu, H.; Huang, C.; Wang, F. A Biocompatible and PH-Responsive Nanohydrogel Based on Cellulose Nanocrystal for Enhanced Toxic Reactive Oxygen Species Generation. Carbohydr. Polym. 2021, 258, 117685. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yu, S.; Shen, Y.; Wu, H. Facile Synthesis of Self-Dispersed β-Cyclodextrin-Coupled Cellulose Microgel for Sustained Release of Vanillin. Int. J. Biol. Macromol. 2022, 208, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, Q.; Wang, H.; Lu, B.; Huang, B. Controllable Construction of Temperature-Sensitive Supramolecular Hydrogel Based on Cellulose and Cyclodextrin. Polymers 2022, 14, 3801. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sharma, M.; Sridhar, K.; Nayak, P.K.; Inbaraj, B.S. Recent Advances in Cellulose-Based Hydrogels: Food Applications. Foods 2023, 12, 350. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Supramolecular Hydrogels from In Situ Host–Guest Inclusion between Chemically Modified Cellulose Nanocrystals and Cyclodextrin. Biomacromolecules 2013, 14, 871–880. [Google Scholar] [CrossRef]
- Park, E.; Ryu, J.H.; Lee, D.; Lee, H. Freeze–Thawing-Induced Macroporous Catechol Hydrogels with Shape Recovery and Sponge-like Properties. ACS Biomater. Sci. Eng. 2021, 7, 4318–4329. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, M.; Li, R.; Cai, Z.; Zhang, H. Thermostable Physically Crosslinked Cryogel from Carboxymethylated Konjac Glucomannan Fabricated by Freeze-Thawing. Food Hydrocoll. 2022, 122, 107103. [Google Scholar] [CrossRef]
- De France, K.J.; Chan, K.J.W.; Cranston, E.D.; Hoare, T. Enhanced Mechanical Properties in Cellulose Nanocrystal–Poly(Oligoethylene Glycol Methacrylate) Injectable Nanocomposite Hydrogels through Control of Physical and Chemical Cross-Linking. Biomacromolecules 2016, 17, 649–660. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Han, C.-R.; Duan, J.-F.; Xu, F.; Sun, R.-C. Tough Nanocomposite Hydrogels from Cellulose Nanocrystals/Poly(Acrylamide) Clusters: Influence of the Charge Density, Aspect Ratio and Surface Coating with PEG. Cellulose 2014, 21, 541–551. [Google Scholar] [CrossRef]
- Ooi, S.Y.; Ahmad, I.; Amin, M.C.I.M. Cellulose Nanocrystals Extracted from Rice Husks as a Reinforcing Material in Gelatin Hydrogels for Use in Controlled Drug Delivery Systems. Ind. Crops Prod. 2016, 93, 227–234. [Google Scholar] [CrossRef]
- Palaganas, N.B.; Mangadlao, J.D.; de Leon, A.C.C.; Palaganas, J.O.; Pangilinan, K.D.; Lee, Y.J.; Advincula, R.C. 3D Printing of Photocurable Cellulose Nanocrystal Composite for Fabrication of Complex Architectures via Stereolithography. ACS Appl. Mater. Interfaces 2017, 9, 34314–34324. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D Printed Scaffolds with Gradient Porosity Based on a Cellulose Nanocrystal Hydrogel. Nanoscale 2018, 10, 4421–4431. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Wu, Q.; Yue, Y.; Zhang, Q. Application of Rod-Shaped Cellulose Nanocrystals in Polyacrylamide Hydrogels. J. Colloid Interface Sci. 2011, 353, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Q.; Zhang, Q. Dynamic Rheology Studies of in Situ Polymerization Process of Polyacrylamide–Cellulose Nanocrystal Composite Hydrogels. Colloid Polym. Sci. 2011, 289, 247–255. [Google Scholar] [CrossRef]
- Hebeish, A.; Farag, S.; Sharaf, S.; Shaheen, T.I. Thermal Responsive Hydrogels Based on Semi Interpenetrating Network of Poly(NIPAm) and Cellulose Nanowhiskers. Carbohydr. Polym. 2014, 102, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Eyley, S.; Thielemans, W. Surface Modification of Cellulose Nanocrystals. Nanoscale 2014, 6, 7764–7779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihranyan, A. Viscoelastic Properties of Cross-Linked Polyvinyl Alcohol and Surface-Oxidized Cellulose Whisker Hydrogels. Cellulose 2013, 20, 1369–1376. [Google Scholar] [CrossRef]
- Mauricio, M.R.; da Costa, P.G.; Haraguchi, S.K.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Synthesis of a Microhydrogel Composite from Cellulose Nanowhiskers and Starch for Drug Delivery. Carbohydr. Polym. 2015, 115, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Foston, M.; Ragauskas, A.J. Improving the Mechanical and Thermal Properties of Gelatin Hydrogels Cross-Linked by Cellulose Nanowhiskers. Carbohydr. Polym. 2013, 91, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lan, Y.; Guo, R.; Zhang, Y.; Xue, W.; Zhang, Y. In Vitro and In Vivo Evaluation of a Novel Collagen/Cellulose Nanocrystals Scaffold for Achieving the Sustained Release of Basic Fibroblast Growth Factor. J. Biomater. Appl. 2015, 29, 882–893. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-Grafted Cellulose Nanocrystals as Cross-Linkers for Bionanocomposite Hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Xu, F.; Sun, R.-C. Revealing Strong Nanocomposite Hydrogels Reinforced by Cellulose Nanocrystals: Insight into Morphologies and Interactions. ACS Appl. Mater. Interfaces 2013, 5, 12960–12967. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Synthesis and Characterization of Mechanically Flexible and Tough Cellulose Nanocrystals–Polyacrylamide Nanocomposite Hydrogels. Cellulose 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.-J.; Zhang, X.-M. Modification of Cellulose Nanocrystal-Reinforced Composite Hydrogels: Effects of Co-Crosslinked and Drying Treatment. Cellulose 2014, 21, 3487–3496. [Google Scholar] [CrossRef]
- Atifi, S.; Su, S.; Hamad, W.Y. Mechanically Tunable Nanocomposite Hydrogels Based on Functionalized Cellulose Nanocrystals. Nord. Pulp Pap. Res. J. 2014, 29, 95–104. [Google Scholar] [CrossRef]
- Yang, D.; Peng, X.; Zhong, L.; Cao, X.; Chen, W.; Wang, S.; Liu, C.; Sun, R. Fabrication of a Highly Elastic Nanocomposite Hydrogel by Surface Modification of Cellulose Nanocrystals. RSC Adv. 2015, 5, 13878–13885. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.-R.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Cellulose Nanocrystals Mechanical Reinforcement in Composite Hydrogels with Multiple Cross-Links: Correlations between Dissipation Properties and Deformation Mechanisms. Macromolecules 2014, 47, 4077–4086. [Google Scholar] [CrossRef]
- Chau, M.; De France, K.J.; Kopera, B.; Machado, V.R.; Rosenfeldt, S.; Reyes, L.; Chan, K.J.W.; Förster, S.; Cranston, E.D.; Hoare, T.; et al. Composite Hydrogels with Tunable Anisotropic Morphologies and Mechanical Properties. Chem. Mater. 2016, 28, 3406–3415. [Google Scholar] [CrossRef]
- Köhnke, T.; Elder, T.; Theliander, H.; Ragauskas, A.J. Ice Templated and Cross-Linked Xylan/Nanocrystalline Cellulose Hydrogels. Carbohydr. Polym. 2014, 100, 24–30. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Silva, M.; Gershovich, P.; Betta, S.; Babo, P.; Caridade, S.G.; Mano, J.F.; Motta, A.; Reis, R.L.; Gomes, M.E. Development of Injectable Hyaluronic Acid/Cellulose Nanocrystals Bionanocomposite Hydrogels for Tissue Engineering Applications. Bioconjug. Chem. 2015, 26, 1571–1581. [Google Scholar] [CrossRef]
- Yang, X.; Bakaic, E.; Hoare, T.; Cranston, E.D. Injectable Polysaccharide Hydrogels Reinforced with Cellulose Nanocrystals: Morphology, Rheology, Degradation, and Cytotoxicity. Biomacromolecules 2013, 14, 4447–4455. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.; Xu, F.; Sun, R. Simple Approach to Reinforce Hydrogels with Cellulose Nanocrystals. Nanoscale 2014, 6, 5934. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, H.; Basri, M.; Rembon, F.; Ramadhan, L.O.A.; Nurdin, M. High Performance of Chitosan-Co-Polyacrylamide-TiO2 Crosslinked Glutaraldehyde Hydrogel as Soil Conditioner for Soybean Plant (Glycine max). Soil Sci. Annu. 2020, 71, 194–204. [Google Scholar] [CrossRef]
- Cho, H.; Yoo, W.-J.; Ahn, J.; Chun, S.-J.; Lee, S.-Y.; Gwon, J. Preparation and Characterization of Cellulose Nanocrystals Reinforced Poly (Vinyl Alcohol) Based Hydrogels for Drug Delivery System. J. Korean Wood Sci. Technol. 2020, 48, 431–449. [Google Scholar] [CrossRef]
- Zhu, Q.; Teng, J.; Liu, X.; Lan, Y.; Guo, R. Preparation and Characterization of Gentamycin Sulfate-Impregnated Gelatin Microspheres/Collagen–Cellulose/Nanocrystal Scaffolds. Polym. Bull. 2018, 75, 77–91. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Wang, B.; Sui, X.; Wang, L.; Yan, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Self-Healing and Injectable Polysaccharide Hydrogels with Tunable Mechanical Properties. Cellulose 2018, 25, 559–571. [Google Scholar] [CrossRef]
- Mihranyan, A.; Edsman, K.; Strømme, M. Rheological Properties of Cellulose Hydrogels Prepared from Cladophora Cellulose Powder. Food Hydrocoll. 2007, 21, 267–272. [Google Scholar] [CrossRef]
- Shin, M.; Shin, S.-H.; Lee, M.; Kim, H.J.; Jeong, J.H.; Choi, Y.H.; Oh, D.X.; Park, J.; Jeon, H.; Eom, Y. Rheological Criteria for Distinguishing Self-Healing and Non-Self-Healing Hydrogels. Polymer 2021, 229, 123969. [Google Scholar] [CrossRef]
- Hou, K.; Li, Y.; Liu, Y.; Zhang, R.; Hsiao, B.S.; Zhu, M. Continuous Fabrication of Cellulose Nanocrystal/Poly(Ethylene Glycol) Diacrylate Hydrogel Fiber from Nanocomposite Dispersion: Rheology, Preparation and Characterization. Polymer 2017, 123, 55–64. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.C.; Song, K.; Cheng, H.N.; Wu, Q. Morphological Influence of Cellulose Nanoparticles (CNs) from Cottonseed Hulls on Rheological Properties of Polyvinyl Alcohol/CN Suspensions. Carbohydr. Polym. 2016, 153, 445–454. [Google Scholar] [CrossRef] [Green Version]
- da Costa, L.M.; Hayaki, S.; Stoyanov, S.R.; Gusarov, S.; Tan, X.; Gray, M.R.; Stryker, J.M.; Tykwinski, R.; de Carneiro, J.W.M.; Sato, H.; et al. 3D-RISM-KH molecular theory of solvation and density functional theory investigation of the role of water in the aggregation of model asphaltenes. Phys. Chem. Chem. Phys. 2012, 14, 3922–3934. [Google Scholar] [CrossRef]
- Honorato-Rios, C.; Kuhnhold, A.; Bruckner, J.R.; Dannert, R.; Schilling, T.; Lagerwall, J.P.F. Equilibrium Liquid Crystal Phase Diagrams and Detection of Kinetic Arrest in Cellulose Nanocrystal Suspensions. Front. Mater. 2016, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Drozdov, A.D.; Christiansen, J.d. Nanocomposite Gels with Permanent and Transient Junctions under Cyclic Loading. Macromolecules 2018, 51, 1462–1473. [Google Scholar] [CrossRef]
- Moud, A.A.; Arjmand, M.; Liu, J.; Yang, Y.; Sanati-Nezhad, A.; Hejazi, S.H. Cellulose nanocrystal structure in the presence of salts. Cellulose 2019, 26, 9387–9401. [Google Scholar] [CrossRef]
- Kjellander, R.; Greberg, H. Mechanisms behind concentration profiles illustrated by charge and concentration distributions around ions in double layers. J. Electroanal. Chem. 1998, 450, 233–251. [Google Scholar] [CrossRef]
- Fiorin, G.; Klein, M.L.; Hénin, J. Using collective variables to drive molecular dynamics simulations. Mol. Phys. 2013, 111, 3345–3362. [Google Scholar] [CrossRef]
- Lorentz, H.A. Ueber die Anwendung des Satzes vom Virial in der kinetischen Theorie der Gase. Ann. Phys. 2010, 248, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Large-Scale Atomic/Molecular Massively Parallel Simulator (LAMMPS). Available online: https://www.lammps.org/#gsc.tab=0 (accessed on 14 June 2023).
- Visual Molecular Dynamics (VMD). Available online: https://www.ks.uiuc.edu/Research/vmd/ (accessed on 14 June 2023).
- Jiang, Y.; Zhou, J.; Feng, C.; Shi, H.; Zhao, G.; Bian, Y. Rheological behavior, 3D printability and the formation of scaffolds with cellulose nanocrystals/gelatin hydrogels. J. Mater. Sci. 2020, 55, 15709–15725. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Zhong, J.; Wang, Y.; Liu, X.; Qin, X. Effect of cellulose nanocrystals on the formation and stability of oil-in-water emulsion formed by octenyl succinic anhydride starch. LWT—Food Sci. Technol. 2021, 151, 112214. [Google Scholar] [CrossRef]
- Esmaeili, M.; George, K.; Rezvan, G.; Taheri-Qazvini, N.; Zhang, R.; Sadati, M. Capillary Flow Characterizations of Chiral Nematic Cellulose Nanocrystal Suspensions. Langmuir 2022, 38, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E. Yet Another Scientific Artificial Reality Application (YASARA). Available online: http://www.yasara.org/ (accessed on 14 June 2023).
- Morais, E.S.; Silva, N.H.C.S.; Sintra, T.E.; Santos, S.A.O.; Neves, B.M.; Almeida, I.F.; Costa, P.C.; Correia-Sá, I.; Ventura, S.P.M.; Silvestre, A.J.D.; et al. Anti-Inflammatory and Antioxidant Nanostructured Cellulose Membranes Loaded with Phenolic-Based Ionic Liquids for Cutaneous Application. Carbohydr. Polym. 2019, 206, 187–197. [Google Scholar] [CrossRef]
- Pacheco, G.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial Cellulose Skin Masks-Properties and Sensory Tests. J. Cosmet. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Bang, N. The Characteristics of Bacterial Nanocellulose Gel Releasing Silk Sericin for Facial Treatment. BMC Biotechnol. 2014, 14, 104. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; He, H.; Wan, R.; Yang, Q.; Luo, H.; Li, L.; Xiong, L. Cellulose Nanocrystals for Skin Barrier Protection by Preparing a Versatile Foundation Liquid. ACS Omega 2021, 6, 2906–2915. [Google Scholar] [CrossRef]
- Kang, L.; Chen, P.; Wang, B.; Jia, J.; Li, J.; Zeng, J.; Cheng, Z.; Gao, W.; Xu, J.; Chen, K. Cellulose Nanocrystal Dye as Reinforcement Matrix of Lipstick for Inhibiting Color Migration. Cellulose 2020, 27, 905–913. [Google Scholar] [CrossRef]
- Awan, F.; Islam, M.S.; Ma, Y.; Yang, C.; Shi, Z.; Berry, R.M.; Tam, K.C. Cellulose Nanocrystal–ZnO Nanohybrids for Controlling Photocatalytic Activity and UV Protection in Cosmetic Formulation. ACS Omega 2018, 3, 12403–12411. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.D.; Hexiu, J.; Patel, D.K.; Ganguly, K.; Lim, K.-T. 3D-Printed Bioactive and Biodegradable Hydrogel Scaffolds of Alginate/Gelatin/Cellulose Nanocrystals for Tissue Engineering. Int. J. Biol. Macromol. 2021, 167, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, L.; Zhang, Z.; Li, Z. Cellulose Nanocrystals Reinforced Gelatin/Bioactive Glass Nanocomposite Scaffolds for Potential Application in Bone Regeneration. J. Biomater. Sci. Polym. Ed. 2020, 31, 984–998. [Google Scholar] [CrossRef]
- De France, K.J.; Badv, M.; Dorogin, J.; Siebers, E.; Panchal, V.; Babi, M.; Moran-Mirabal, J.; Lawlor, M.; Cranston, E.D.; Hoare, T. Tissue Response and Biodistribution of Injectable Cellulose Nanocrystal Composite Hydrogels. ACS Biomater. Sci. Eng. 2019, 5, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Shin, W.-C.; Ganguly, K.; Lim, K.-T. Fabrication and Characterization of 3D Printable Nanocellulose-Based Hydrogels for Tissue Engineering. RSC Adv. 2021, 11, 7466–7478. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Shi, H.; Zhao, G.; Zhang, Q.; Feng, C.; Xv, X. Preparation of Cellulose Nanocrystal/Oxidized Dextran/Gelatin (CNC/OD/GEL) Hydrogels and Fabrication of a CNC/OD/GEL Scaffold by 3D Printing. J. Mater. Sci. 2020, 55, 2618–2635. [Google Scholar] [CrossRef]
- Abbasi Moud, A.; Sanati-Nezhad, A.; Hejazi, S.H. Confocal Analysis of Cellulose Nanocrystal (CNC) Based Hydrogels and Suspensions. Cellulose 2021, 28, 10259–10276. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.F.; Cranston, E.D.; Rojas, O.J. Porous Nanocellulose Gels and Foams: Breakthrough Status in the Development of Scaffolds for Tissue Engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a Versatile Platform: From the Delivery of Active Molecules to Tissue Engineering Applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.J.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-Linked Cellulose Nanocrystal Aerogels as Viable Bone Tissue Scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Tersur Orasugh, J.; Dutta, S.; Das, D.; Nath, J.; Pal, C.; Chattopadhyay, D. Utilization of Cellulose Nanocrystals (CNC) Biopolymer Nanocomposites in Ophthalmic Drug Delivery System (ODDS). J. Nanotechnol. Res. 2019, 1, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Boonlai, W.; Tantishaiyakul, V.; Hirun, N. Characterization of Κ-carrageenan/Methylcellulose/Cellulose Nanocrystal Hydrogels for 3D Bioprinting. Polym. Int. 2022, 71, 181–191. [Google Scholar] [CrossRef]
- Goodarzi, K.; Jonidi Shariatzadeh, F.; Solouk, A.; Akbari, S.; Mirzadeh, H. Injectable Drug Loaded Gelatin Based Scaffolds as Minimally Invasive Approach for Drug Delivery System: CNC/PAMAM Nanoparticles. Eur. Polym. J. 2020, 139, 109992. [Google Scholar] [CrossRef]
- Åhlén, M.; Tummala, G.K.; Mihranyan, A. Nanoparticle-Loaded Hydrogels as a Pathway for Enzyme-Triggered Drug Release in Ophthalmic Applications. Int. J. Pharm. 2018, 536, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.L.; Abou-Elesoud, W.S.; Safwat, E.M.; Hassan, E.A.; Fadel, S.M.; Labeeb, A.M. Effect of Cellulose Nanocrystals on Rheology, Liquid Crystal, and Delivery Behavior of Metronidazole Poloxamer-Based in-Situ Dental Medication. Cellulose 2022, 29, 9511–9529. [Google Scholar] [CrossRef]
- Wang, F.; Huang, K.; Xu, Z.; Shi, F.; Chen, C. Self-Healable Nanocellulose Composite Hydrogels Combining Multiple Dynamic Bonds for Drug Delivery. Int. J. Biol. Macromol. 2022, 203, 143–152. [Google Scholar] [CrossRef]
- Orasugh, J.T.; Sarkar, G.; Saha, N.R.; Das, B.; Bhattacharyya, A.; Das, S.; Mishra, R.; Roy, I.; Chattoapadhyay, A.; Ghosh, S.K.; et al. Effect of Cellulose Nanocrystals on the Performance of Drug Loaded In Situ Gelling Thermo-Responsive Ophthalmic Formulations. Int. J. Biol. Macromol. 2019, 124, 235–245. [Google Scholar] [CrossRef]
- Yusefi, M.; Soon, M.L.-K.; Teow, S.-Y.; Monchouguy, E.I.; Neerooa, B.N.H.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J.; Shameli, K. Fabrication of Cellulose Nanocrystals as Potential Anticancer Drug Delivery Systems for Colorectal Cancer Treatment. Int. J. Biol. Macromol. 2022, 199, 372–385. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Zhang, D.; Wu, X.; Zhao, Y.; Shang, L.; Ren, J.; Zhao, Y. Natural Polysaccharide Based Complex Drug Delivery System from Microfluidic Electrospray for Wound Healing. Appl. Mater. Today 2021, 23, 101000. [Google Scholar] [CrossRef]
- Afrin, S.; Shahruzzaman, M.; Haque, P.; Islam, M.S.; Hossain, S.; Rashid, T.U.; Ahmed, T.; Takafuji, M.; Rahman, M.M. Advanced CNC/PEG/PDMAA Semi-IPN Hydrogel for Drug Delivery Management in Wound Healing. Gels 2022, 8, 340. [Google Scholar] [CrossRef]
- Jeong, D.I.; Kim, S.; Kim, M.-H.; Yoon, I.-S.; Lee, S.-H.; Kim, D.-D.; Cho, H.-J. Donepezil Hydrochloride-Reinforced Cellulose Nanocrystal-Aggregated Gel Structure for Long-Acting Drug Delivery. Carbohydr. Polym. 2022, 296, 119887. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered Nanocellulose-Based Hydrogels for Smart Drug Delivery Applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef]
- Hasan, N.; Rahman, L.; Kim, S.-H.; Cao, J.; Arjuna, A.; Lallo, S.; Jhun, B.H.; Yoo, J.-W. Recent Advances of Nanocellulose in Drug Delivery Systems. J. Pharm. Investig. 2020, 50, 553–572. [Google Scholar] [CrossRef]
- He, P.; Dai, L.; Wei, J.; Zhu, X.; Li, J.; Chen, Z.; Ni, Y. Nanocellulose-Based Hydrogels as Versatile Drug Delivery Vehicles: A Review. Int. J. Biol. Macromol. 2022, 222, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Melinda, F.; Chawla, P. A Review on Antifungal Efficiency of Plant Extracts Entrenched Polysaccharide-Based Nanohydrogels. Nutrients 2021, 13, 2055. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.-T.; Phan, T.-H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.-S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef] [PubMed]
- Madhusudana Rao, K.; Kumar, A.; Han, S.S. Polysaccharide Based Bionanocomposite Hydrogels Reinforced with Cellulose Nanocrystals: Drug Release and Biocompatibility Analyses. Int. J. Biol. Macromol. 2017, 101, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.R.; Babo, P.S.; Gulino, M.; Costa, L.; Oliveira, J.M.; Silva-Correia, J.; Domingues, R.M.A.; Reis, R.L.; Gomes, M.E. Injectable and Tunable Hyaluronic Acid Hydrogels Releasing Chemotactic and Angiogenic Growth Factors for Endodontic Regeneration. Acta Biomater. 2018, 77, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Mariia, K.; Arif, M.; Shi, J.; Song, F.; Chi, Z.; Liu, C. Novel Chitosan-Ulvan Hydrogel Reinforcement by Cellulose Nanocrystals with Epidermal Growth Factor for Enhanced Wound Healing: In Vitro and In Vivo Analysis. Int. J. Biol. Macromol. 2021, 183, 435–446. [Google Scholar] [CrossRef]
- Maestri, C.A.; Motta, A.; Moschini, L.; Bernkop-Schnürch, A.; Baus, R.A.; Lecca, P.; Scarpa, M. Composite Nanocellulose-based Hydrogels with Spatially Oriented Degradation and Retarded Release of Macromolecules. J. Biomed. Mater. Res. Part A 2020, 108, 1509–1519. [Google Scholar] [CrossRef]
- Bertsch, P.; Schneider, L.; Bovone, G.; Tibbitt, M.W.; Fischer, P.; Gstöhl, S. Injectable Biocompatible Hydrogels from Cellulose Nanocrystals for Locally Targeted Sustained Drug Release. ACS Appl. Mater. Interfaces 2019, 11, 38578–38585. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Lim, K.-T. Multifunctional Bioactive Chitosan/Cellulose Nanocrystal Scaffolds Eradicate Bacterial Growth and Sustain Drug Delivery. Int. J. Biol. Macromol. 2021, 170, 178–188. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic Nanocellulose Alginate Hydrogel Beads as Potential Drug Delivery System. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Khan, A.; Wen, Y.; Huq, T.; Ni, Y. Cellulosic Nanomaterials in Food and Nutraceutical Applications: A Review. J. Agric. Food Chem. 2018, 66, 8–19. [Google Scholar] [CrossRef]
- Xiao, Y.; Kang, S.; Liu, Y.; Guo, X.; Li, M.; Xu, H. Effect and Mechanism of Calcium Ions on the Gelation Properties of Cellulose Nanocrystals-Whey Protein Isolate Composite Gels. Food Hydrocoll. 2021, 111, 106401. [Google Scholar] [CrossRef]
- Jin, X.; Qu, R.; Wang, Y.; Li, D.; Wang, L. Effect and Mechanism of Acid-Induced Soy Protein Isolate Gels as Influenced by Cellulose Nanocrystals and Microcrystalline Cellulose. Foods 2022, 11, 461. [Google Scholar] [CrossRef]
- Armstrong, C.D.; Yue, L.; Deng, Y.; Qi, H.J. Enabling Direct Ink Write Edible 3D Printing of Food Purees with Cellulose Nanocrystals. J. Food Eng. 2022, 330, 111086. [Google Scholar] [CrossRef]
- Abitbol, T.; Mijlkovic, A.; Malafronte, L.; Stevanic, J.S.; Larsson, P.T.; Lopez-Sanchez, P. Cellulose Nanocrystal/Low Methoxyl Pectin Gels Produced by Internal Ionotropic Gelation. Carbohydr. Polym. 2021, 260, 117345. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic Stabilization of Emulsions and Emulsion Gels with Water-Soluble Polymers and Cellulose Nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Xu, H.; Hao, Z.; Gao, J.; Zhou, Q.; Li, W.; Liao, X.; Zheng, M.; Zhou, Y.; Yu, Z.; Song, C.; et al. Complexation between Rice Starch and Cellulose Nanocrystal from Black Tea Residues: Gelatinization Properties and Digestibility In Vitro. Int. J. Biol. Macromol. 2023, 234, 123695. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Cao, J.; Jiang, W. Improving the Performance of Edible Food Packaging Films by Using Nanocellulose as an Additive. Int. J. Biol. Macromol. 2021, 166, 288–296. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind. Eng. Chem. Res. 2017, 56, 4718–4735. [Google Scholar] [CrossRef]
- Mu, R.; Hong, X.; Ni, Y.; Li, Y.; Pang, J.; Wang, Q.; Xiao, J.; Zheng, Y. Recent Trends and Applications of Cellulose Nanocrystals in Food Industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.; Yang, Y.; Pang, B.; Xu, W.; Duan, G.; Jiang, S.; Zhang, K. Recent Progress on Nanocellulose Aerogels: Preparation, Modification, Composite Fabrication, Applications. Adv. Mater. 2021, 33, 2005569. [Google Scholar] [CrossRef]
- Perumal, A.B.; Nambiar, R.B.; Moses, J.A.; Anandharamakrishnan, C. Nanocellulose: Recent Trends and Applications in the Food Industry. Food Hydrocoll. 2022, 127, 107484. [Google Scholar] [CrossRef]
- Guo, K.; Wei, P.; Xie, Y.; Huang, X. Smart Ultra-Stable Foams Stabilized Using Cellulose Nanocrystal (CNC) Gels via Noncovalent Bonding. Chem. Commun. 2022, 58, 4723–4726. [Google Scholar] [CrossRef]
- Su, E.; Li, Q.; Xu, M.; Yuan, Y.; Wan, Z.; Yang, X.; Binks, B.P. Highly Stable and Thermo-Responsive Gel Foams by Synergistically Combining Glycyrrhizic Acid Nanofibrils and Cellulose Nanocrystals. J. Colloid Interface Sci. 2021, 587, 797–809. [Google Scholar] [CrossRef]
- Hu, Z.; Ballinger, S.; Pelton, R.; Cranston, E.D. Surfactant-Enhanced Cellulose Nanocrystal Pickering Emulsions. J. Colloid Interface Sci. 2015, 439, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Cui, R.; Lu, S.; Hu, X.; Xu, B.; Song, Y.; Hu, X. High Internal Phase Pickering Emulsions Stabilized by Cellulose Nanocrystals for 3D Printing. Food Hydrocoll. 2022, 125, 107418. [Google Scholar] [CrossRef]




| Components | Notes | Cargo | Application | Ref. |
|---|---|---|---|---|
| CNCs; 5-aminolevulinic acid (ALA); dopamine (DPA) | Increased adhesion of the nanohydrogels to cells, and reactive oxygen species (ROS) production | Paclitaxel (PTX) | Cancer therapy | [67] |
| poly(ε-caprolactone-co-lactide)-b-poly(ethylene glycol)-b-poly(ε-caprolactone-co-lactide (PCLA); CNCs | Good injectability and high shape fidelity in 3D printing | DOX | Cancer therapy | [150] |
| Xanthan (XG); Chitosan (CS); CNCs | Improved mechanical performance | 5-Flurouracil | Tissue engineering Drug delivery | [151] |
| hyaluronic acid (HA); CNCs; Enriched with platelet lysate | Enhanced cells’ viability and angiogenic activity | Chemotactic and pro-angiogenic growth factors | Tissue regeneration | [152] |
| Chitosan-ulvan hydrogel; CNCs | Fast wound-healing efficiency | Epidermal growth factor (EGF) | Wound healing | [153] |
| CNCs decorated with Fe3O4 nanoparticles; poly(N-isopropylacrylamide) (PNIPAm) | High drug-loading content (10.18 g/g) Thermo-response triggered by NIR | Vancomycin | Wound healing | [65] |
| CS; CNCs | Hydrogel scaffold degrades according to a preferred route | Bovine serum albumin (BSA) | Release of macromolecules | [154] |
| CNC gels formed by salt-induced charge screening | Drug release modulated by the incorporation of sucrose or xanthan gum | BSA; Tetracycline (TC); Doxorubicin (DOX) | Drug delivery | [155] |
| CS; CNCs | Improved cell viability and mineralization Enhanced osteogenic-related gene expression Enhanced antibacterial activity | TC | Osteogenesis; Antibacterial agent; Drug delivery | [156] |
| Magnetic nanocellulose (m-CNCs) alginate hydrogel beads | m-CNCs increased the integrity and the swelling percentage | Ibuprofen | Drug delivery | [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, S.R.S.; Azevedo, A.G.; Teixeira, P.F.; Fernandes, C.B.P. Cellulose Nanocrystal (CNC) Gels: A Review. Gels 2023, 9, 574. https://doi.org/10.3390/gels9070574
Veloso SRS, Azevedo AG, Teixeira PF, Fernandes CBP. Cellulose Nanocrystal (CNC) Gels: A Review. Gels. 2023; 9(7):574. https://doi.org/10.3390/gels9070574
Chicago/Turabian StyleVeloso, Sérgio R. S., Ana G. Azevedo, Paulo F. Teixeira, and Célio B. P. Fernandes. 2023. "Cellulose Nanocrystal (CNC) Gels: A Review" Gels 9, no. 7: 574. https://doi.org/10.3390/gels9070574
APA StyleVeloso, S. R. S., Azevedo, A. G., Teixeira, P. F., & Fernandes, C. B. P. (2023). Cellulose Nanocrystal (CNC) Gels: A Review. Gels, 9(7), 574. https://doi.org/10.3390/gels9070574