Rheological Characterization of Three-Dimensional Neuronal Cultures Embedded in PEGylated Fibrin Hydrogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Hydrogels’ Viscoelastic Behavior
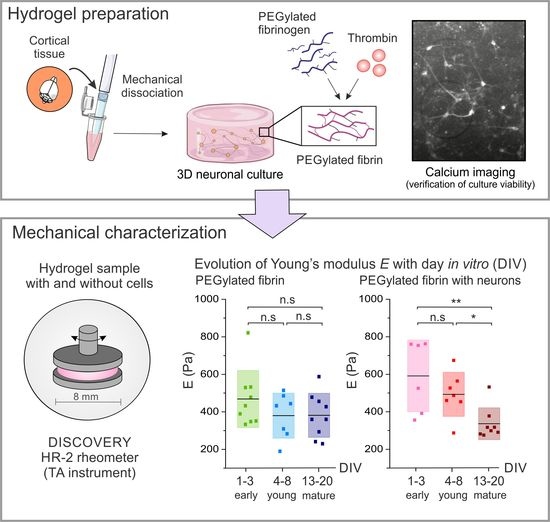
2.2. Time Evolution of PEGylated Fibrin Hydrogels
2.3. Immunostaining Results
2.4. Discussion
3. Conclusions
4. Materials and Methods
4.1. Hydrogels Preparation
4.2. Hydrogels Rheological Characterization
4.2.1. Determination of Rheological Properties: Data Analysis
4.2.2. Rheological Tests
- Time sweep: This rheological test was used to track the evolution of the hydrogel structure along time and procure information such as the degradation, gelation or solvent evaporation. The oscillation frequency, , and the strain amplitude, , were kept constant in this test. In the present study, they took values of rad/s (1 Hz) and , respectively (see Table 1).
- Strain sweep: The amplitude of the strain oscillation, , is changed periodically while the frequency, , remains constant (see Figure 1b). This test is performed to obtain information about the linear viscoelastic region (LVR). As a consequence of the linear response and the small deformations, the test can be carried out without damaging the microscopic structure of the sample, which is crucial to keep the scaffold and the inner neural network intact. For these experiments, was fixed to rad/s (1 Hz) and was progressively increased from a to strain.
- Frequency sweep: The oscillation frequency, , is progressively increased at a constant strain amplitude (see Figure 1b). This test provides information about the rheological response of the hydrogel at different timescales and reveals whether the sample softens or thickens at faster deformations. The tests are performed at a selected , ensuring that the sample remains in the LVR. For the present work, was fixed at 5%, and increased from to 100 rad/s.
4.3. Immunochemistry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two dimensional |
| 3D | Three dimensional |
| ECM | Extracellular matrix |
| AAV | Adeno-associated virus |
| DIV | Day in vitro |
| MEM | Modified Eagle Medium |
| E16 | Embryonic day 16 |
| PEG | Polyethylene glycol |
| PEG-NHS | Mono-Methyl polyethylene glycol succinate N-succinimidyl ester |
| PDMS | Polydimethylsiloxane |
| SAOS | Small Amplitude Oscillatory Shearing |
| LVR | Linear viscoelastic regime |
| Storage modulus | |
| Loss modulus | |
| Complex shear modulus | |
| Phase shift | |
| E | Young’s modulus |
| Poisson’s ratio | |
| Oscillation frequency | |
| Strain amplitude | |
| PBS | Phosphate-buffered saline |
| RPM | Revolutions per minute |
References
- Bang, S.; Hwang, K.S.; Jeong, S.; Cho, I.J.; Choi, N.; Kim, J.; Kim, H.N. Engineered neural circuits for modeling brain physiology and neuropathology. Acta Biomater. 2021, 132, 379–400. [Google Scholar] [CrossRef]
- Weir, J.S.; Christiansen, N.; Sandvig, A.; Sandvig, I. Selective inhibition of excitatory synaptic transmission alters the emergent bursting dynamics of in vitro neural networks. Front. Neural Circuits 2023, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Tetzlaff, C.; Okujeni, S.; Egert, U.; Wörgötter, F.; Butz, M. Self-organized criticality in developing neuronal networks. PLoS Comput. Biol. 2010, 6, e1001013. [Google Scholar] [CrossRef] [Green Version]
- Meunier, D.; Lambiotte, R.; Bullmore, E.T. Modular and hierarchically modular organization of brain networks. Front. Neurosci. 2010, 4, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raggenbass, M. Vasopressin-and oxytocin-induced activity in the central nervous system: Electrophysiological studies using in-vitro systems. Prog. Neurobiol. 2001, 64, 307–326. [Google Scholar] [CrossRef]
- Chiappalone, M.; Bove, M.; Vato, A.; Tedesco, M.; Martinoia, S. Dissociated cortical networks show spontaneously correlated activity patterns during in vitro development. Brain Res. 2006, 1093, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Teller, S.; Estévez-Priego, E.; Granell, C.; Tornero, D.; Andilla, J.; Olarte, O.E.; Loza-Alvarez, P.; Arenas, A.; Soriano, J. Spontaneous functional recovery after focal damage in neuronal cultures. Eneuro 2020, 7, 1. [Google Scholar] [CrossRef]
- Teller, S.; Tahirbegi, I.B.; Mir, M.; Samitier, J.; Soriano, J. Magnetite-Amyloid-β deteriorates activity and functional organization in an in vitro model for Alzheimer’s disease. Sci. Rep. 2015, 5, 17261. [Google Scholar] [CrossRef] [Green Version]
- Stiso, J.; Bassett, D.S. Spatial Embedding Imposes Constraints on Neuronal Network Architectures. Trends Cogn. Sci. 2018, 22, 1127–1142. [Google Scholar] [CrossRef]
- Schwartz, E.; Nenning, K.H.; Heuer, K.; Jeffery, N.; Bertrand, O.C.; Toro, R.; Kasprian, G.; Prayer, D.; Langs, G. Evolution of cortical geometry and its link to function, behaviour and ecology. Nat. Commun. 2023, 14, 2252. [Google Scholar] [CrossRef]
- Wagenaar, D.A.; Pine, J.; Potter, S.M. An extremely rich repertoire of bursting patterns during the development of cortical cultures. BMC Neurosci. 2006, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.R.; D’Esposito, M. The segregation and integration of distinct brain networks and their relationship to cognition. J. Neurosci. 2016, 36, 12083–12094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, J.G.; Soriano, J.; Alvarez-Lacalle, E.; Teller, S.; Casademunt, J. Noise focusing and the emergence of coherent activity in neuronal cultures. Nat. Phys. 2013, 9, 582–590. [Google Scholar] [CrossRef]
- Tang-Schomer, M.D.; White, J.D.; Tien, L.W.; Schmitt, L.I.; Valentin, T.M.; Graziano, D.J.; Hopkins, A.M.; Omenetto, F.G.; Haydon, P.G.; Kaplan, D.L. Bioengineered functional brain-like cortical tissue. Proc. Natl. Acad. Sci. USA 2014, 111, 13811–13816. [Google Scholar] [CrossRef]
- Bourke, J.L.; Quigley, A.F.; Duchi, S.; O’Connell, C.D.; Crook, J.M.; Wallace, G.G.; Cook, M.J.; Kapsa, R.M. Three-dimensional neural cultures produce networks that mimic native brain activity. J. Tissue Eng. Regen. Med. 2018, 12, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Chwalek, K.; Sood, D.; Cantley, W.L.; White, J.D.; Tang-Schomer, M.; Kaplan, D.L. Engineered 3D silk-collagen-based model of polarized neural tissue. JoVE J. Vis. Exp. 2015, 104, e52970. [Google Scholar]
- Lam, D.; Fischer, N.O.; Enright, H.A. Probing function in 3D neuronal cultures: A survey of 3D multielectrode array advances. Curr. Opin. Pharmacol. 2021, 60, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Dingle, Y.T.L.; Liaudanskaya, V.; Finnegan, L.T.; Berlind, K.C.; Mizzoni, C.; Georgakoudi, I.; Nieland, T.J.; Kaplan, D.L. Functional characterization of three-dimensional cortical cultures for in vitro modeling of brain networks. IScience 2020, 23, 101434. [Google Scholar] [CrossRef] [PubMed]
- Rabadan, M.A.; De La Cruz, E.D.; Rao, S.B.; Chen, Y.; Gong, C.; Crabtree, G.; Xu, B.; Markx, S.; Gogos, J.A.; Yuste, R.; et al. An in vitro model of neuronal ensembles. Nat. Commun. 2022, 13, 3340. [Google Scholar] [CrossRef]
- Frega, M.; Tedesco, M.; Massobrio, P.; Pesce, M.; Martinoia, S. Network dynamics of 3D engineered neuronal cultures: A new experimental model for in-vitro electrophysiology. Sci. Rep. 2014, 4, 5489. [Google Scholar] [CrossRef] [Green Version]
- Marder, E. Variability, compensation, and modulation in neurons and circuits. Proc. Natl. Acad. Sci. USA 2011, 108, 15542–15548. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. R. Soc. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef] [Green Version]
- Ozgun, A.; Lomboni, D.; Arnott, H.; Staines, W.A.; Woulfe, J.; Variola, F. Biomaterials-based strategies for in vitro neural models. Biomater. Sci. 2022, 10, 1134–1165. [Google Scholar] [CrossRef] [PubMed]
- Javier-Torrent, M.; Zimmer-Bensch, G.; Nguyen, L. Mechanical forces orchestrate brain development. Trends Neurosci. 2021, 44, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.W.; Cua, R.; Keough, M.B.; Haylock-Jacobs, S.; Yong, V.W. Pathophysiology of the brain extracellular matrix: A new target for remyelination. Nat. Rev. Neurosci. 2013, 14, 722–729. [Google Scholar] [CrossRef]
- Lam, D.; Enright, H.A.; Peters, S.K.; Moya, M.L.; Soscia, D.A.; Cadena, J.; Alvarado, J.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Optimizing cell encapsulation condition in ECM-Collagen I hydrogels to support 3D neuronal cultures. J. Neurosci. Methods 2020, 329, 108460. [Google Scholar] [CrossRef]
- Pan, L.; Ren, Y.; Cui, F.; Xu, Q. Viability and differentiation of neural precursors on hyaluronic acid hydrogel scaffold. J. Neurosci. Res. 2009, 87, 3207–3220. [Google Scholar] [CrossRef]
- Wu, S.; Xu, R.; Duan, B.; Jiang, P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B 2017, 5, 3870–3878. [Google Scholar] [CrossRef]
- Spearman, B.S.; Agrawal, N.K.; Rubiano, A.; Simmons, C.S.; Mobini, S.; Schmidt, C.E. Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.T.; Di Lisa, D.; Massobrio, P.; Colistra, N.; Pesce, M.; Catelani, T.; Dellacasa, E.; Raiteri, R.; Martinoia, S.; Pastorino, L. Soft chitosan microbeads scaffold for 3D functional neuronal networks. Biomaterials 2018, 156, 159–171. [Google Scholar] [CrossRef]
- Cao, Z.; Gilbert, R.J.; He, W. Simple Agarose- Chitosan gel composite system for enhanced neuronal growth in three dimensions. Biomacromolecules 2009, 10, 2954–2959. [Google Scholar] [CrossRef]
- Rowe, S.L.; Lee, S.; Stegemann, J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007, 3, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadashzadeh, A.; Moghassemi, S.; Amorim, C. Evaluation of PEGylated fibrin as a three-dimensional biodegradable scaffold for ovarian tissue engineering. Mater. Today Chem. 2021, 22, 100626. [Google Scholar] [CrossRef]
- Galler, K.M.; Cavender, A.C.; Koeklue, U.; Suggs, L.J.; Schmalz, G.; D’Souza, R.N. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regen. Med. 2011, 6, 191–200. [Google Scholar] [CrossRef]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between structure and rheology of hydrogels for various applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Oswald, P. Rheophysics: The Deformation and Flow of Matter; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Zuidema, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Zhang, G.; Drinnan, C.T.; Geuss, L.R.; Suggs, L.J. Vascular differentiation of bone marrow stem cells is directed by a tunable three-dimensional matrix. Acta Biomater. 2010, 6, 3395–3403. [Google Scholar] [CrossRef]
- Ghosh, K.; Shu, X.Z.; Mou, R.; Lombardi, J.; Prestwich, G.D.; Rafailovich, M.H.; Clark, R.A.F. Rheological Characterization of in Situ Cross-Linkable Hyaluronan Hydrogels. Biomacromolecules 2005, 6, 2857–2865. [Google Scholar] [CrossRef]
- Krieger, I.M. Bingham Award Lecture—1989: The role of instrument inertia in controlled-stress rheometers. J. Rheol. 1990, 34, 471–483. [Google Scholar] [CrossRef]
- Ewoldt, R.; Johnston, M.; Caretta, L. Experimental challenges of shear rheology: How to avoid bad data. In Complex Fluids in Biological Systems; Spagnolie, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Pasut, G.; Veronese, F.M. PEGylation of proteins as tailored chemistry for optimized bioconjugates. In Polymer Therapeutics I; Springer: Berlin/Heidelberg, Germany, 2006; pp. 95–134. [Google Scholar]
- Mosesson, M.W. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 2005, 3, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Blombäck, B.; Bark, N. Fibrinopeptides and fibrin gel structure. Biophys. Chem. 2004, 112, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Fee, C. Protein PEGylation: An overview of chemistry and process considerations. Eur. Pharm. Rev. 2010, 15, 18–26. [Google Scholar]
- Liu, H.; Collins, S.F.; Suggs, L.J. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials 2006, 27, 6004–6014. [Google Scholar] [CrossRef]
- Lam, D.; Enright, H.A.; Cadena, J.; Peters, S.K.; Sales, A.P.; Osburn, J.J.; Soscia, D.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Tissue-specific extracellular matrix accelerates the formation of neural networks and communities in a neuron-glia co-culture on a multi-electrode array. Sci. Rep. 2019, 9, 4159. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Gong, J.; Yang, X. Substrate stiffness in nerve cells. Brain Sci. Adv. 2023, 9, 24–34. [Google Scholar] [CrossRef]
- Budday, S.; Sommer, G.; Birkl, C.; Langkammer, C.; Haybaeck, J.; Kohnert, J.; Bauer, M.; Paulsen, F.; Steinmann, P.; Kuhl, E.; et al. Mechanical characterization of human brain tissue. Acta Biomater. 2017, 48, 319–340. [Google Scholar] [CrossRef]
- Kim, H.N.; Choi, N. Consideration of the Mechanical Properties of Hydrogels for Brain Tissue Engineering and Brain-on-a-chip. Biochip J. 2019, 13, 8–19. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Samanipour, R.; Tahmooressi, H.; Rezaei Nejad, H.; Hirano, M.; Shin, S.R.; Hoorfar, M. A review on 3D printing functional brain model. Biomicrofluidics 2022, 16, 011501. [Google Scholar] [CrossRef]
- Tibau, E.; Ludl, A.A.; Rudiger, S.; Orlandi, J.G.; Soriano, J. Neuronal Spatial Arrangement Shapes Effective Connectivity Traits of in vitro Cortical Networks. IEEE Trans. Netw. Sci. Eng. 2020, 7, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Montalà-Flaquer, M.; López-León, C.F.; Tornero, D.; Houben, A.M.; Fardet, T.; Monceau, P.; Bottani, S.; Soriano, J. Rich dynamics and functional organization on topographically designed neuronal networks in vitro. Iscience 2022, 25, 105680. [Google Scholar] [CrossRef]
- Estévez-Priego, E.; Moreno-Fina, M.; Monni, E.; Kokaia, Z.; Soriano, J.; Tornero, D. Long-term calcium imaging reveals functional development in hiPSC-derived cultures comparable to human but not rat primary cultures. Stem Cell Rep. 2023, 18, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Spedden, E.; White, J.D.; Naumova, E.N.; Kaplan, D.L.; Staii, C. Elasticity maps of living neurons measured by combined fluorescence and atomic force microscopy. Biophys. J. 2012, 103, 868–877. [Google Scholar] [CrossRef] [Green Version]
- Low, L.K.; Cheng, H.J. Axon pruning: An essential step underlying the developmental plasticity of neuronal connections. Philos. Trans. R. Soc. Biol. Sci. 2006, 361, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Ylä-Outinen, L.; Rangasami, V.K.; Narkilahti, S.; Oommen, O.P. Bidirectional cell-matrix interaction dictates neuronal network formation in a brain-mimetic 3D scaffold. Acta Biomater. 2022, 140, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.M.; Rodrigues, G.M.; Kulkarni, R.U.; Rao, A.T.; Chernavsky, N.E.; Miller, E.W.; Schaffer, D.V. Efficient generation of hPSC-derived midbrain dopaminergic neurons in a fully defined, scalable, 3D biomaterial platform. Sci. Rep. 2017, 7, 40573. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.N.; Smith, C.K.; Patrick, C.W., Jr. Rheological and recovery properties of poly (ethylene glycol) diacrylate hydrogels and human adipose tissue. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2005, 73, 313–319. [Google Scholar] [CrossRef]
- Oyen, M. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Duong, H.; Wu, B.; Tawil, B. Modulation of 3D fibrin matrix stiffness by intrinsic fibrinogen–thrombin compositions and by extrinsic cellular activity. Tissue Eng. Part A 2009, 15, 1865–1876. [Google Scholar] [CrossRef]
- Janmey, P.A.; Georges, P.C.; Hvidt, S. Basic rheology for biologists. Methods Cell Biol. 2007, 83, 3–27. [Google Scholar] [PubMed]
- Divoux, T.; Barentin, C.; Manneville, S. From stress-induced fluidization processes to Herschel-Bulkley behaviour in simple yield stress fluids. Soft Matter. 2011, 7, 8409–8418. [Google Scholar] [CrossRef] [Green Version]






| Parameter | Time Sweep | Strain Test | Frequency Sweep |
|---|---|---|---|
| Frequency (rad/s) | 0.1–100 | ||
| Strain (%) | 5 | 0.1–100 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-León, C.F.; Soriano, J.; Planet, R. Rheological Characterization of Three-Dimensional Neuronal Cultures Embedded in PEGylated Fibrin Hydrogels. Gels 2023, 9, 642. https://doi.org/10.3390/gels9080642
López-León CF, Soriano J, Planet R. Rheological Characterization of Three-Dimensional Neuronal Cultures Embedded in PEGylated Fibrin Hydrogels. Gels. 2023; 9(8):642. https://doi.org/10.3390/gels9080642
Chicago/Turabian StyleLópez-León, Clara F., Jordi Soriano, and Ramon Planet. 2023. "Rheological Characterization of Three-Dimensional Neuronal Cultures Embedded in PEGylated Fibrin Hydrogels" Gels 9, no. 8: 642. https://doi.org/10.3390/gels9080642
APA StyleLópez-León, C. F., Soriano, J., & Planet, R. (2023). Rheological Characterization of Three-Dimensional Neuronal Cultures Embedded in PEGylated Fibrin Hydrogels. Gels, 9(8), 642. https://doi.org/10.3390/gels9080642