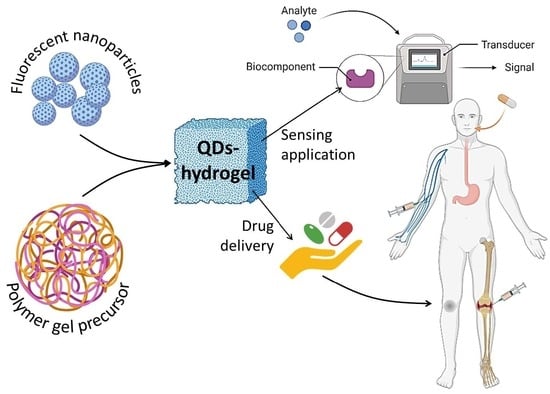
Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications
Abstract
:1. Introduction
2. Classification of Fluorescent Nanoparticles
3. Fabrication of Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels
In Situ Gelation Technique
4. Infusion of QDs into Hydrogel Technique
5. Low-Molecular-Weight Polymer-QDs’ Assembly
6. Biomedical Significance Properties of the QDs-Polymer Hydrogels
7. Biosensing Applications
| Sensing Target | Sensor | Gel Matrix | Detection Limit | Ref. |
|---|---|---|---|---|
| Cr(VI) | CDs | Lignin+CNF | ~11 mg/L | [132] |
| Cr(VI) | CDs | PVP | 1.2 µM | [133] |
| Ag+ | CDs | BMIM-BF4 IL | 0.55 µg/mL | [98] |
| Fe3+ | CDs | MCC | 65 µM | [134] |
| NO2- | Doped CDs | Agarose | 0.018 µM | [135] |
| TC | CDs | Sodium alginate | 2 µM | [136] |
| Glucose | CDs | PAA | 0.04 µM | [125] |
| Bacillus and Staphylococcus strains | CDs | DTG | 105 cells/mL | [137] |
| Progesterone | CdSe/CdS/ZnS | PEG | 55 µM | [138] |
| Dopamine | CdTe | MSA | 50 nmol/L | [127] |
| Uric acid | CdS | GPTMS | 50 µM | [139] |
| Fe3+ | CdTe | PEGDA+ HMP | 14 nM | [113] |
| Polyaromatic compounds | GQDs | AETA+MBA | - | [140] |
| Laccase | S,N-codoped GQDs | NC | 0.048 U/mL | [141] |
8. Drug Delivery Applications
9. Emerging Trends and Future Directions for Research
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhattacharya, S.; Samanta, S.K. Soft-Nanocomposites of Nanoparticles and Nanocarbons with Supramolecular and Polymer Gels and Their Applications. Chem. Rev. 2016, 116, 11967–12028. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Li, H.; He, B.; Dang, F.; Lin, J.; Fan, R.; Hou, C.; Liu, H.; Zhang, J.; Ma, Y.; et al. Bio-gel derived nickel/carbon nanocomposites with enhanced microwave absorption. J. Mater. Chem. C 2018, 6, 8812–8822. [Google Scholar] [CrossRef]
- Sheng, Y.; Yang, J.; Wang, F.; Liu, L.; Liu, H.; Yan, C.; Guo, Z. Sol-gel synthesized hexagonal boron nitride/titania nanocomposites with enhanced photocatalytic activity. Appl. Surf. Sci. 2019, 465, 154–163. [Google Scholar] [CrossRef]
- Xie, P.; Li, Y.; Hou, Q.; Sui, K.; Liu, C.; Fu, X.; Zhang, J.; Murugadoss, V.; Fan, J.; Wang, Y.; et al. Tunneling-induced negative permittivity in Ni/MnO nanocomposites by a bio-gel derived strategy. J. Mater. Chem. C 2020, 8, 3029–3039. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef]
- Mohaghegh, N.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Najafabadi, A.H.; Zehtabi, F.; Kouchehbaghi, N.H.; Sharma, S.; Markowska-Szczupak, A.; Kowalska, E. Apatite-coated Ag/AgBr/TiO2 nanocomposites: Insights into the antimicrobial mechanism in the dark and under visible-light irradiation. Appl. Surf. Sci. 2023, 617, 156574. [Google Scholar] [CrossRef]
- Shaheen, S.; Saeed, Z.; Ahmad, A.; Pervaiz, M.; Younas, U.; Khan, R.R.M.; Luque, R.; Rajendran, S. Green synthesis of graphene-based metal nanocomposite for electro and photocatalytic activity; recent advancement and future prospective. Chemosphere 2022, 311, 136982. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.D.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Amirjani, A.; Amlashi, N.B.; Ahmadiani, Z.S. Plasmon-Enhanced Photocatalysis Based on Plasmonic Nanoparticles for Energy and Environmental Solutions: A Review. ACS Appl. Nano Mater. 2023, 6, 9085–9123. [Google Scholar] [CrossRef]
- Fan, X.; Liu, J.; Song, Z.; Han, X.; Deng, Y.; Zhong, C.; Hu, W. Porous nanocomposite gel polymer electrolyte with high ionic conductivity and superior electrolyte retention capability for long-cycle-life flexible zinc–air batteries. Nano Energy 2019, 56, 454–462. [Google Scholar] [CrossRef]
- Pastoriza-Santos, I.; Kinnear, C.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Plasmonic polymer nanocomposites. Nat. Rev. Mater. 2018, 3, 375–391. [Google Scholar] [CrossRef]
- Lee, C.Y.; Park, K.S.; Jung, Y.K.; Park, H.G. A label-free fluorescent assay for deoxyribonuclease I activity based on DNA-templated silver nanocluster/graphene oxide nanocomposite. Biosens. Bioelectron. 2017, 93, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Song, P.; Gu, H.; Ma, C.; Guo, Y.; Zhang, H.; Xu, X.; Zhang, Q.; Gu, J. Nanopolydopamine coupled fluorescent nanozinc oxide reinforced epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 126–136. [Google Scholar] [CrossRef]
- Wang, C.; Shen, W.; Rong, Z.; Liu, X.; Gu, B.; Xiao, R.; Wang, S. Layer-by-layer assembly of magnetic-core dual quantum dot-shell nanocomposites for fluorescence lateral flow detection of bacteria. Nanoscale 2020, 12, 795–807. [Google Scholar] [CrossRef] [PubMed]
- Kulal, P.; Badalamoole, V. Efficient removal of dyes and heavy metal ions from waste water using Gum ghatti-graft-poly(4-acryloylmorpholine) hydrogel incorporated with magnetite nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104207. [Google Scholar] [CrossRef]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Sun, A.; He, X.; Ji, X.; Hu, D.; Pan, M.; Zhang, L.; Qian, Z. Current research progress of photopolymerized hydrogels in tissue engineering. Chin. Chem. Lett. 2021, 32, 2117–2126. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Ehrenhofer, A.; Elstner, M.; Wallmersperger, T. Normalization of hydrogel swelling behavior for sensoric and actuatoric applications. Sens. Actuators B Chem. 2018, 255, 1343–1353. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. 3D printed magnetic polymer composite hydrogels for hyperthermia and magnetic field driven structural manipulation. Prog. Polym. Sci. 2022, 131, 101574. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Golmohammadi, H.; Abbasi-Moayed, S.; Nejad, M.A.F.; Fahimi-Kashani, N.; Jafarinejad, S.; Shahrajabian, M.; Hormozi-Nezhad, M.R. Nanoparticle-based optical sensor arrays. Nanoscale 2017, 9, 16546–16563. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, B.E.; Blakey, I.; Squires, O.; Peng, H.; Boase, N.R.B.; Alexander, C.; Parsons, P.G.; Boyle, G.M.; Whittaker, A.K.; Thurecht, K.J. Multimodal Polymer Nanoparticles with Combined 19F Magnetic Resonance and Optical Detection for Tunable, Targeted, Multimodal Imaging In Vivo. J. Am. Chem. Soc. 2014, 136, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Abelha, T.F.; Neumann, P.R.; Holthof, J.; Dreiss, C.A.; Alexander, C.; Green, M.; Dailey, L.A. Low molecular weight PEG–PLGA polymers provide a superior matrix for conjugated polymer nanoparticles in terms of physicochemical properties, biocompatibility and optical/photoacoustic performance. J. Mater. Chem. B 2019, 7, 5115–5124. [Google Scholar] [CrossRef]
- Sun, C.; Du, K.; Fang, C.; Bhattarai, N.; Veiseh, O.; Kievit, F.; Stephen, Z.; Lee, D.; Ellenbogen, R.G.; Ratner, B.; et al. PEG-Mediated Synthesis of Highly Dispersive Multifunctional Superparamagnetic Nanoparticles: Their Physicochemical Properties and Function In Vivo. ACS Nano 2010, 4, 2402–2410. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, Z.; Elsayed-Ali, H.E.; Wang, H.; Henry, L.L.; Wang, Q.; Zou, S.; Zhang, T. Identification of single nanoparticles. Nanoscale 2011, 3, 31–44. [Google Scholar] [CrossRef]
- Patel, M.; Patel, R.; Rai, M. Nanomedicines. In Nanotechnology in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2021; pp. 1–28. [Google Scholar]
- Samuel, M.S.; Ravikumar, M.; John, J.A.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Jain, A.; Ranjan, S.; Dasgupta, N.; Ramalingam, C. Nanomaterials in food and agriculture: An overview on their safety concerns and regulatory issues. Crit. Rev. Food Sci. Nutr. 2018, 58, 297–317. [Google Scholar] [CrossRef]
- Li, X.; Xu, W.; Xin, Y.; Yuan, J.; Ji, Y.; Chu, S.; Liu, J.; Luo, Q. Supramolecular Polymer Nanocomposites for Biomedical Applications. Polymers 2021, 13, 513. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Bioimaging probes based on magneto-fluorescent nanoparticles. Pharmaceutics 2023, 15, 686. [Google Scholar] [CrossRef]
- Amirjani, A.; Tsoulos, T.V.; Sajjadi, S.H.; Antonucci, A.; Wu, S.-J.; Tagliabue, G.; Haghshenas, D.F.; Boghossian, A.A. Plasmon-induced near-infrared fluorescence enhancement of single-walled carbon nanotubes. Carbon 2022, 194, 162–175. [Google Scholar] [CrossRef]
- Chimene, D.; Alge, D.L.; Gaharwar, A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015, 27, 7261–7284. [Google Scholar] [CrossRef] [PubMed]
- Alibakhshi, A.; Abarghooi Kahaki, F.; Ahangarzadeh, S.; Yaghoobi, H.; Yarian, F.; Arezumand, R.; Ranjbari, J.; Mokhtarzadeh, A.; de la Guardia, M. Targeted cancer therapy through antibody fragments-decorated nanomedicines. J. Control. Release 2017, 268, 323–334. [Google Scholar] [CrossRef]
- Xie, S.; Ai, L.; Cui, C.; Fu, T.; Cheng, X.; Qu, F.; Tan, W. Functional Aptamer-Embedded Nanomaterials for Diagnostics and Therapeutics. ACS Appl. Mater. Interfaces 2021, 13, 9542–9560. [Google Scholar] [CrossRef]
- Ansari, A.A.; Aldajani, K.M.; AlHazaa, A.N.; Albrithen, H.A. Recent progress of fluorescent materials for fingermarks detection in forensic science and anti-counterfeiting. Coord. Chem. Rev. 2022, 462, 214523. [Google Scholar] [CrossRef]
- O’Farrell, A.C.; Shnyder, S.D.; Marston, G.; Coletta, P.L.; Gill, J.H. Non-invasive molecular imaging for preclinical cancer therapeutic development. Br. J. Pharmacol. 2013, 169, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of Hydrogels: From Synthetic Strategies, Classification, and Properties to Biomedical Applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-I.; Park, S.-Y. Smart fluorescent hydrogel glucose biosensing microdroplets with dual-mode fluorescence quenching and size reduction. ACS Appl. Mater. Interfaces 2018, 10, 30172–30179. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, Z.; Su, Z.; Zhang, K.; Ren, J.; Sun, R.; Wang, X. All-biomass fluorescent hydrogels based on biomass carbon dots and alginate/nanocellulose for biosensing. ACS Appl. Bio Mater. 2018, 1, 1398–1407. [Google Scholar] [CrossRef]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Whiteside, M.D.; Werner, G.D.; Caldas, V.E.; van’t Padje, A.; Dupin, S.E.; Elbers, B.; Bakker, M.; Wyatt, G.A.; Klein, M.; Hink, M.A. Mycorrhizal fungi respond to resource inequality by moving phosphorus from rich to poor patches across networks. Curr. Biol. 2019, 29, 2043–2050.e8. [Google Scholar] [CrossRef] [PubMed]
- Drbohlavova, J.; Adam, V.; Kizek, R.; Hubalek, J. Quantum dots—Characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Winnik, F.M.; Maysinger, D. Quantum dot cytotoxicity and ways to reduce it. Acc. Chem. Res. 2013, 46, 672–680. [Google Scholar] [CrossRef]
- Xu, J.; Jie, X.; Xie, F.; Yang, H.; Wei, W.; Xia, Z. Flavonoid moiety-incorporated carbon dots for ultrasensitive and highly selective fluorescence detection and removal of Pb2+. Nano Res. 2018, 11, 3648–3657. [Google Scholar] [CrossRef]
- Tao, S.; Feng, T.; Zheng, C.; Zhu, S.; Yang, B. Carbonized polymer dots: A brand new perspective to recognize luminescent carbon-based nanomaterials. J. Phys. Chem. Lett. 2019, 10, 5182–5188. [Google Scholar] [CrossRef]
- Li, S.; Peng, Z.; Dallman, J.; Baker, J.; Othman, A.M.; Blackwelder, P.L.; Leblanc, R.M. Crossing the blood–brain–barrier with transferrin conjugated carbon dots: A zebrafish model study. Colloids Surf. B Biointerfaces 2016, 145, 251–256. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. Fluorescent quantum dots-based hydrogels: Synthesis, Fabrication and multimodal biosensing. Talanta Open 2023, 8, 100243. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saravanan, A.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Naturally derived carbon dots in situ confined self-healing and breathable hydrogel monolith for anomalous diffusion-driven phytomedicine release. ACS Appl. Bio Mater. 2022, 5, 5617–5633. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Ahmed, S.R.; Sherazee, M.; Margel, S.; Gedanken, A.; Srinivasan, S.; Rajabzadeh, A.R. Carbon dot biopolymer-based flexible functional films for antioxidant and food monitoring applications. ACS Appl. Polym. Mater. 2022, 4, 9323–9340. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Saha, A.; Noked, M.; Margel, S.; Gedanken, A. Carbon-dots-initiated photopolymerization: An in situ synthetic approach for MXene/poly (norepinephrine)/copper hybrid and its application for mitigating water pollution. ACS Appl. Mater. Interfaces 2021, 13, 31038–31050. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiao, F.; Xu, H. Fluorescent detection of emerging virus based on nanoparticles: From synthesis to application. TrAC Trends Anal. Chem. 2023, 161, 116999. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, P.; Wang, S.; Melnykov, A.; Jiang, Q.; Seth, A.; Wang, Z.; Morrissey, J.J.; George, I.; Gandra, S. Ultrasensitive lateral-flow assays via plasmonically active antibody-conjugated fluorescent nanoparticles. Nat. Biomed. Eng. 2023. [Google Scholar] [CrossRef]
- Yan, R.; Wen, Z.; Hu, X.; Wang, W.; Meng, H.; Song, Y.; Wang, S.; Tang, Y. A sensitive sensing system based on fluorescence dipeptide nanoparticles for sulfadimethoxine determination. Food Chem. 2023, 405, 134963. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Ganguly, S.; Margel, S.; Gedanken, A. Immobilization of heteroatom-doped carbon dots onto nonpolar plastics for antifogging, antioxidant, and food monitoring applications. Langmuir 2021, 37, 3508–3520. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Maruthapandi, M.; Das, P.; Ganguly, S.; Margel, S.; Luong, J.H.; Gedanken, A. Applications of N-doped carbon dots as antimicrobial agents, antibiotic carriers, and selective fluorescent probes for nitro explosives. ACS Appl. Bio Mater. 2020, 3, 8023–8031. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Agarwal, T.; Maity, P.; Ghosh, S.; Choudhary, S.; Gangopadhyay, S.; Maiti, T.K.; Dhara, S.; Banerjee, S. Heteroatom doped blue luminescent carbon dots as a nano-probe for targeted cell labeling and anticancer drug delivery vehicle. Mater. Chem. Phys. 2019, 237, 121860. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Saha, A.; Noked, M.; Gedanken, A.; Margel, S. Mussel-inspired polynorepinephrine/MXene-based magnetic nanohybrid for electromagnetic interference shielding in X-band and strain-sensing performance. Langmuir 2022, 38, 3936–3950. [Google Scholar] [CrossRef]
- Ganguly, S.; Kanovsky, N.; Das, P.; Gedanken, A.; Margel, S. Photopolymerized thin coating of polypyrrole/graphene nanofiber/iron oxide onto nonpolar plastic for flexible electromagnetic radiation shielding, strain sensing, and non-contact heating applications. Adv. Mater. Interfaces 2021, 8, 2101255. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Bose, M.; Ray, D.; Ghosh, S.; Mondal, S.; Aswal, V.K.; Das, A.K.; Banerjee, S.; Das, N.C. Surface quaternized nanosensor as a one-arrow-two-hawks approach for fluorescence turn “on–off–on” bifunctional sensing and antibacterial activity. New J. Chem. 2019, 43, 6205–6219. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Bose, M.; Mondal, S.; Das, A.K.; Das, N. Strongly blue-luminescent N-doped carbogenic dots as a tracer metal sensing probe in aqueous medium and its potential activity towards in situ Ag-nanoparticle synthesis. Sens. Actuators B Chem. 2017, 252, 735–746. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Ganguly, S. Introduction to polymer composite-based sensors. In Polymeric Nanocomposite Materials for Sensor Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–21. [Google Scholar]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Maity, P.P.; Ghosh, S.; Dhara, S.; Das, N.C. Design of psyllium-g-poly(acrylic acid-co-sodium acrylate)/cloisite 10A semi-IPN nanocomposite hydrogel and its mechanical, rheological and controlled drug release behaviour. Int. J. Biol. Macromol. 2018, 111, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Lin, S.; Zhao, X. Hydrogel machines. Mater. Today 2020, 36, 102–124. [Google Scholar] [CrossRef]
- Xue, J.; Zou, Y.; Deng, Y.; Li, Z. Bioinspired sensor system for health care and human-machine interaction. EcoMat 2022, 4, e12209. [Google Scholar] [CrossRef]
- Ou, P.; Wu, J.; Lin, Y.; Tan, X.; Wu, Y.; Chen, Z.; Wei, F.; Huang, K. Flexible photoelectrochemical sensor for highly sensitive chloramphenicol detection based on M-TiO2-CdTe QDs/CdS QDs composite. Anal. Bioanal. Chem. 2022, 414, 2065–2078. [Google Scholar] [CrossRef]
- Pan, S.; Gayathri, G.; Reshma, T.; Mangamma, G.; Prasad, A.K.; Das, A. A sensitive humidity sensor at low pressure with SnO2 QDs. Sens. Actuators A Phys. 2022, 346, 113835. [Google Scholar] [CrossRef]
- He, K.; Yu, X.; Qin, L.; Wu, Y. CdS QDs: Facile synthesis, design and application as an “on–off” sensor for sensitive and selective monitoring Cu2+, Hg2+ and Mg2+ in foods. Food Chem. 2022, 390, 133116. [Google Scholar] [CrossRef]
- Abbass, R.; Alkaaby, H.H.C.; Kadhim, Z.J.; Izzat, S.E.; Kadhim, A.A.; Adhab, A.H.; Pakravan, P. Using the aluminum decorated graphitic-C3N4 quantum dote (QD) as a sensor, sorbent, and photocatalyst for artificial photosynthesis; a DFT study. J. Mol. Graph. Model. 2022, 117, 108302. [Google Scholar] [CrossRef]
- Riccò, R.; Nizzero, S.; Penna, E.; Meneghello, A.; Cretaio, E.; Enrichi, F. Ultra-small dye-doped silica nanoparticles via modified sol-gel technique. J. Nanoparticle Res. 2018, 20, 117. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Y.; Cui, Q.; Peng, R.; Liu, R.; Cao, Q.; Li, L. Fluorescent Nanoparticles Synthesized by Carbon-Nitride-Stabilized Pickering Emulsion Polymerization for Targeted Cancer Cell Imaging. ACS Appl. Bio Mater. 2019, 2, 5127–5135. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, B.; Liu, M.; Liu, W.; Chen, Y.; Wei, Y. Fabrication of aggregation induced emission dye-based fluorescent organic nanoparticles via emulsion polymerization and their cell imaging applications. Polym. Chem. 2014, 5, 399–404. [Google Scholar] [CrossRef]
- Liao, B.; Wang, W.; Long, P.; He, B.; Li, F.; Liu, Q. Synthesis of fluorescent carbon nanoparticles grafted with polystyrene and their fluorescent fibers processed by electrospinning. RSC Adv. 2014, 4, 57683–57690. [Google Scholar] [CrossRef]
- Yang, Z.; Fan, W.; Zou, J.; Tang, W.; Li, L.; He, L.; Shen, Z.; Wang, Z.; Jacobson, O.; Aronova, M.A.; et al. Precision Cancer Theranostic Platform by In Situ Polymerization in Perylene Diimide-Hybridized Hollow Mesoporous Organosilica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 14687–14698. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Itzhaki, E.; Hadad, E.; Gedanken, A.; Margel, S. Microwave-Synthesized Polysaccharide-Derived Carbon Dots as Therapeutic Cargoes and Toughening Agents for Elastomeric Gels. ACS Appl. Mater. Interfaces 2020, 12, 51940–51951. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, K.; Cheng, L.; Liu, Z.; Lee, S.-T.; Liu, J. Specific Detection and Simultaneously Localized Photothermal Treatment of Cancer Cells Using Layer-by-Layer Assembled Multifunctional Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 6443–6452. [Google Scholar] [CrossRef]
- Shaabani, E.; Sharifiaghdam, M.; De Keersmaecker, H.; De Rycke, R.; De Smedt, S.; Faridi-Majidi, R.; Braeckmans, K.; Fraire, J.C. Layer by Layer Assembled Chitosan-Coated Gold Nanoparticles for Enhanced siRNA Delivery and Silencing. Int. J. Mol. Sci. 2021, 22, 831. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Zabolotskii, A.A.; Pereverzev, N.V.; Bespalova, I.I.; Yefimova, S.L.; Malyukin, Y.V.; Plekhanov, A.I. Metal-Enhanced Fluorescence of Pseudoisocyanine J-Aggregates Formed in Layer-by-Layer Assembled Films. J. Phys. Chem. C 2015, 119, 2743–2751. [Google Scholar] [CrossRef]
- Hu, M.; Gu, X.; Hu, Y.; Deng, Y.; Wang, C. PVA/Carbon Dot Nanocomposite Hydrogels for Simple Introduction of Ag Nanoparticles with Enhanced Antibacterial Activity. Macromol. Mater. Eng. 2016, 301, 1352–1362. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Zhang, M.; Wang, W.; Shen, J.; Ye, Z.; Zhou, N. Injectable in situ self-cross-linking hydrogels based on hemoglobin, carbon quantum dots, and sodium alginate for real-time detection of wound bacterial infection and efficient postoperative prevention of tumor recurrence. Langmuir 2020, 36, 13263–13273. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-A.; Jang, E.; Koh, W.-G.; Kim, B. Development of analytic microdevices for the detection of phenol using polymer hydrogel particles containing enzyme–QD conjugates. Talanta 2011, 84, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, H. Highly Fluorescent Fluoride-Responsive Hydrogels Embedded with CdTe Quantum Dots. ACS Appl. Mater. Interfaces 2012, 4, 721–724. [Google Scholar] [CrossRef]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of polymer hydrogels and nanoparticulate systems for biomedical and pharmaceutical applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [PubMed]
- Liras, M.; Quijada-Garrido, I.; García, O. QDs decorated with thiol-monomer ligands as new multicrosslinkers for the synthesis of smart luminescent nanogels and hydrogels. Polym. Chem. 2017, 8, 5317–5326. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, S.; Beines, P.W.; Ding, N.; Jakubowicz, P.; Knoll, W. Rapid and highly efficient preparation of water-soluble luminescent quantum dots via encapsulation by thermo-and redox-responsive hydrogels. Chem. Mater. 2008, 20, 7215–7219. [Google Scholar] [CrossRef]
- Ahirwar, R.C.; Mehra, S.; Reddy, S.M.; Alshamsi, H.A.; Kadhem, A.A.; Karmankar, S.B.; Sharma, A. Progression of Quantum Dots Confined Polymeric Systems for Sensorics. Polymers 2023, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S. 10 Quantum Dots–Rubber Composites. In Quantum Dots and Polymer Nanocomposites: Synthesis, Chemistry, and Applications; CRC Press: Boca Raton, FL, USA, 2022; p. 189. [Google Scholar]
- Shen, X.; Xu, W.; Ouyang, J.; Na, N. Fluorescence resonance energy transfer-based nanomaterials for the sensing in biological systems. Chin. Chem. Lett. 2022, 33, 4505–4516. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Zhang, Y.; Zhao, P.; Cai, J.; Yao, Y.; Liang, J. Preparation of molecularly imprinted cysteine modified zinc sulfide quantum dots based sensor for rapid detection of dopamine hydrochloride. Molecules 2023, 28, 3646. [Google Scholar] [CrossRef]
- Yukawa, H.; Sato, K.; Baba, Y. Theranostics applications of quantum dots in regenerative medicine, cancer medicine, and infectious diseases. Adv. Drug Deliv. Rev. 2023, 200, 114863. [Google Scholar] [CrossRef]
- Barettin, D. State of the Art of Continuous and Atomistic Modeling of Electromechanical Properties of Semiconductor Quantum Dots. Nanomaterials 2023, 13, 1820. [Google Scholar] [CrossRef]
- Su, H.; Wang, W.; Shi, R.; Tang, H.; Sun, L.; Wang, L.; Liu, Q.; Zhang, T. Recent advances in quantum dot catalysts for hydrogen evolution: Synthesis, characterization, and photocatalytic application. Carbon Energy 2023, e280. [Google Scholar] [CrossRef]
- Ganguly, S. 13 Challenges Prospects of and Magnetic Future Quantum Dots. In Magnetic Quantum Dots for Bioimaging; CRC Press: Boca Raton, FL, USA, 2023; p. 269. [Google Scholar]
- Quaranta, A.; Carturan, S.; Campagnaro, A.; Dalla Palma, M.; Giarola, M.; Daldosso, N.; Maggioni, G.; Mariotto, G. Highly fluorescent xerogels with entrapped carbon dots for organic scintillators. Thin Solid Film. 2014, 553, 188–192. [Google Scholar] [CrossRef]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 41, 5116–5118. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.; Valcárcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Sahub, C.; Andrews, J.L.; Smith, J.P.; Arif, M.A.M.; Tomapatanaget, B.; Steed, J.W. Enhancement of sensitivity for dichlorvos detection by a low-weight gelator based on bolaamphiphile amino acid derivatives decorated with a hybrid graphene quantum dot/enzyme/hydrogel. Mater. Chem. Front. 2021, 5, 6850–6859. [Google Scholar] [CrossRef]
- Cayuela, A.; Kennedy, S.R.; Soriano, M.L.; Jones, C.D.; Valcárcel, M.; Steed, J.W. Fluorescent carbon dot–molecular salt hydrogels. Chem. Sci. 2015, 6, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Das, P.; Das, S.; Ghorai, U.; Bose, M.; Ghosh, S.; Mondal, M.; Das, A.K.; Banerjee, S.; Das, N.C. Microwave assisted green synthesis of Zwitterionic photolumenescent N-doped carbon dots: An efficient ‘on-off’chemosensor for tracer Cr(+6) considering the inner filter effect and nano drug-delivery vector. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123604. [Google Scholar] [CrossRef]
- Sun, X.; Li, G.; Yin, Y.; Zhang, Y.; Li, H. Carbon quantum dot-based fluorescent vesicles and chiral hydrogels with biosurfactant and biocompatible small molecule. Soft Matter 2018, 14, 6983–6993. [Google Scholar] [CrossRef]
- Li, Y.; Männel, M.J.; Hauck, N.; Patel, H.P.; Auernhammer, G.K.; Chae, S.; Fery, A.; Li, J.; Thiele, J. Embedment of quantum dots and biomolecules in a dipeptide hydrogel formed in situ using microfluidics. Angew. Chem. Int. Ed. 2021, 60, 6724–6732. [Google Scholar] [CrossRef]
- Misra, R.; Sharma, A.; Shiras, A.; Gopi, H.N. Backbone engineered γ-peptide amphitropic gels for immobilization of semiconductor quantum dots and 2D cell culture. Langmuir 2017, 33, 7762–7768. [Google Scholar] [CrossRef]
- Hao, C.; Gao, Y.; Wu, D.; Li, S.; Xu, L.; Wu, X.; Guo, J.; Sun, M.; Li, X.; Xu, C. Tailoring chiroptical activity of iron disulfide quantum dot hydrogels with circularly polarized light. Adv. Mater. 2019, 31, 1903200. [Google Scholar] [CrossRef]
- Ganguly, S.; Margel, S. A Review on Synthesis Methods of Phyllosilicate- and Graphene-Filled Composite Hydrogels. J. Compos. Sci. 2022, 6, 15. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.-T.; Kim, J. Polyphenol derived bioactive carbon quantum dot-incorporated multifunctional hydrogels as an oxidative stress attenuator for antiaging and in vivo wound-healing applications. Biomater. Sci. 2022, 10, 3527–3539. [Google Scholar] [CrossRef] [PubMed]
- Gattas-Asfura, K.M.; Zheng, Y.; Micic, M.; Snedaker, M.J.; Ji, X.; Sui, G.; Orbulescu, J.; Andreopoulos, F.M.; Pham, S.M.; Wang, C. Immobilization of quantum dots in the photo-cross-linked poly (ethylene glycol)-based hydrogel. J. Phys. Chem. B 2003, 107, 10464–10469. [Google Scholar] [CrossRef]
- Micic, M.; Zheng, Y.; Moy, V.; Zhang, X.-H.; Andreopoulos, F.M.; Leblanc, R.M. Comparative studies of surface topography and mechanical properties of a new, photo-switchable PEG-based hydrogel. Colloids Surf. B Biointerfaces 2003, 27, 147–158. [Google Scholar] [CrossRef]
- Ru, Y.; Sui, L.; Song, H.; Liu, X.; Tang, Z.; Zang, S.-Q.; Yang, B.; Lu, S. Rational Design of Multicolor-Emitting Chiral Carbonized Polymer Dots for Full-Color and White Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2021, 60, 14091–14099. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, S.; Xu, X.; Zhuang, J.; Zhang, H.; Zhang, X.; Hu, C.; Lei, B.; Kaminski, C.F.; Liu, Y. Carbon Dot-Silica Nanoparticle Composites for Ultralong Lifetime Phosphorescence Imaging in Tissue and Cells at Room Temperature. Chem. Mater. 2019, 31, 9887–9894. [Google Scholar] [CrossRef]
- Joseph, J.; Anappara, A.A. Cool white, persistent room-temperature phosphorescence in carbon dots embedded in a silica gel matrix. Phys. Chem. Chem. Phys. 2017, 19, 15137–15144. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Yang, C. Ratiometric fluorescence sensor for Fe3+ ions detection based on quantum dot-doped hydrogel optical fiber. Sens. Actuators B Chem. 2018, 264, 52–58. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Benitez-Martinez, S.; Valcarcel, M. Photoluminescent sensing hydrogel platform based on the combination of nanocellulose and S, N-codoped graphene quantum dots. Sens. Actuators B Chem. 2017, 245, 946–953. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Ma, C.; Yin, Y.; Sun, C. Carbon quantum dots-based fluorescent hydrogel hybrid platform for sensitive detection of iron ions. J. Chem. 2022, 2022, 3737646. [Google Scholar] [CrossRef]
- Gogoi, N.; Barooah, M.; Majumdar, G.; Chowdhury, D. Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl. Mater. Interfaces 2015, 7, 3058–3067. [Google Scholar] [CrossRef]
- Kharlampieva, E.; Kozlovskaya, V.; Zavgorodnya, O.; Lilly, G.D.; Kotov, N.A.; Tsukruk, V.V. pH-responsive photoluminescent LbL hydrogels with confined quantum dots. Soft Matter 2010, 6, 800–807. [Google Scholar] [CrossRef]
- Luo, Q.; Yuan, H.; Zhang, M.; Jiang, P.; Liu, M.; Xu, D.; Guo, X.; Wu, Y. A 3D porous fluorescent hydrogel based on amino-modified carbon dots with excellent sorption and sensing abilities for environmentally hazardous Cr (VI). J. Hazard. Mater. 2021, 401, 123432. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, L.; Van Vliet, K.; Miserez, A.; Holten-Andersen, N. White Light-Emitting Multistimuli-Responsive Hydrogels with Lanthanides and Carbon Dots. ACS Appl. Mater. Interfaces 2018, 10, 10409–10418. [Google Scholar] [CrossRef] [PubMed]
- Omidi, M.; Yadegari, A.; Tayebi, L. Wound dressing application of pH-sensitive carbon dots/chitosan hydrogel. RSC Adv. 2017, 7, 10638–10649. [Google Scholar] [CrossRef]
- Mohammadi, S.; Mohammadi, S.; Salimi, A. A 3D hydrogel based on chitosan and carbon dots for sensitive fluorescence detection of microRNA-21 in breast cancer cells. Talanta 2021, 224, 121895. [Google Scholar] [CrossRef]
- Artzi, N.; Oliva, N.; Puron, C.; Shitreet, S.; Artzi, S.; Bon Ramos, A.; Groothuis, A.; Sahagian, G.; Edelman, E.R. In vivo and in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat. Mater. 2011, 10, 890. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Xu, F.; Li, Y.; Xu, Z.; Wei, D.; Feng, Y.; Wang, Y.; Jia, D.; Zhou, Y. Visual in vivo degradation of injectable hydrogel by real-time and non-invasive tracking using carbon nanodots as fluorescent indicator. Biomaterials 2017, 145, 192–206. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, K.; Shao, M.; Lu, X.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution and toxicology study of functionalized upconversion nanoparticles in mice. Nanomedicine 2011, 6, 1327–1340. [Google Scholar] [CrossRef]
- Cho, M.-J.; Park, S.-Y. Carbon-dot-based ratiometric fluorescence glucose biosensor. Sens. Actuators B Chem. 2019, 282, 719–729. [Google Scholar] [CrossRef]
- Jang, E.; Son, K.J.; Kim, B.; Koh, W.-G. Phenol biosensor based on hydrogel microarrays entrapping tyrosinase and quantum dots. Analyst 2010, 135, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Wen, D.; Gaponik, N.; Eychmüller, A. Enzyme-Encapsulating Quantum Dot Hydrogels and Xerogels as Biosensors: Multifunctional Platforms for Both Biocatalysis and Fluorescent Probing. Angew. Chem. Int. Ed. 2013, 52, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.Y.; Nam, D.H.; Ryu, J.; Ku, S.H.; Park, C.B. Self-assembled, photoluminescent peptide hydrogel as a versatile platform for enzyme-based optical biosensors. Biosens. Bioelectron. 2011, 26, 1860–1865. [Google Scholar] [CrossRef]
- Li, Y.; Luo, S.; Gui, Y.; Wang, X.; Tian, Z.; Yu, H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors 2023, 13, 287. [Google Scholar] [CrossRef]
- Jang, E.; Kim, S.; Koh, W.-G. Microfluidic bioassay system based on microarrays of hydrogel sensing elements entrapping quantum dot–enzyme conjugates. Biosens. Bioelectron. 2012, 31, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Won, H.J.; Ryplida, B.; Kim, S.G.; Lee, G.; Ryu, J.H.; Park, S.Y. Diselenide-bridged carbon-dot-mediated self-healing, conductive, and adhesive wireless hydrogel sensors for label-free breast cancer detection. ACS Nano 2020, 14, 8409–8420. [Google Scholar] [CrossRef]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr (VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef]
- Truskewycz, A.; Beker, S.A.; Ball, A.S.; Murdoch, B.; Cole, I. Incorporation of quantum carbon dots into a PVP/ZnO hydrogel for use as an effective hexavalent chromium sensing platform. Anal. Chim. Acta 2020, 1099, 126–135. [Google Scholar] [CrossRef]
- Cheng, C.; Xing, M.; Wu, Q. Green synthesis of fluorescent carbon dots/hydrogel nanocomposite with stable Fe3+ sensing capability. J. Alloys Compd. 2019, 790, 221–227. [Google Scholar] [CrossRef]
- Zhan, Y.; Zeng, Y.; Li, L.; Luo, F.; Qiu, B.; Lin, Z.; Guo, L. Ratiometric fluorescent hydrogel test kit for on-spot visual detection of nitrite. ACS Sens. 2019, 4, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Ehtesabi, H.; Roshani, S.; Bagheri, Z.; Yaghoubi-Avini, M. Carbon dots—Sodium alginate hydrogel: A novel tetracycline fluorescent sensor and adsorber. J. Environ. Chem. Eng. 2019, 7, 103419. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Nandi, S.; Jelinek, R. Carbon-dot–hydrogel for enzyme-mediated bacterial detection. RSC Adv. 2017, 7, 588–594. [Google Scholar] [CrossRef]
- Chen, M.; Grazon, C.; Sensharma, P.; Nguyen, T.T.; Feng, Y.; Chern, M.; Baer, R.; Varongchayakul, N.; Cook, K.; Lecommandoux, S. Hydrogel-Embedded Quantum Dot–Transcription Factor Sensors for Quantitative Progesterone Detection. ACS Appl. Mater. Interfaces 2020, 12, 43513–43521. [Google Scholar] [CrossRef] [PubMed]
- Azmi, N.E.; Rashid, A.H.A.; Abdullah, J.; Yusof, N.A.; Sidek, H. Fluorescence biosensor based on encapsulated quantum dots/enzymes/sol-gel for non-invasive detection of uric acid. J. Lumin. 2018, 202, 309–315. [Google Scholar] [CrossRef]
- Martín-Pacheco, A.; Del Río Castillo, A.E.; Martín, C.; Herrero, M.A.A.; Merino, S.; Garcia Fierro, J.L.; Díez-Barra, E.; Vázquez, E. Graphene quantum dot–aerogel: From nanoscopic to macroscopic fluorescent materials. sensing polyaromatic compounds in water. ACS Appl. Mater. Interfaces 2018, 10, 18192–18201. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Benítez-Martínez, S.; Soriano, M.L.; Valcárcel, M. Fluorescent nanocellulosic hydrogels based on graphene quantum dots for sensing laccase. Anal. Chim. Acta 2017, 974, 93–99. [Google Scholar] [CrossRef]
- Yang, S.; Lu, F.; Liu, Y.; Ning, Y.; Tian, S.; Zuo, P.; Ji, X.; He, Z. Quantum dots-based hydrogel microspheres for visual determination of lactate and simultaneous detection coupled with microfluidic device. Microchem. J. 2021, 171, 106801. [Google Scholar] [CrossRef]
- Bui, T.T.; Park, S.-Y. A carbon dot–hemoglobin complex-based biosensor for cholesterol detection. Green Chem. 2016, 18, 4245–4253. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, J.; Chen, Z.; Han, C.; Zhang, X.; Yang, S.; Zhou, P.; Deng, T.; Yu, C. A multilevel fluorometric biosensor based on boric acid embedded in carbon dots to detect intracellular and serum glucose. Sens. Actuators B Chem. 2022, 350, 130898. [Google Scholar] [CrossRef]
- Jamei, H.R.; Rezaei, B.; Ensafi, A.A. Ultra-sensitive and selective electrochemical biosensor with aptamer recognition surface based on polymer quantum dots and C60/MWCNTs-polyethylenimine nanocomposites for analysis of thrombin protein. Bioelectrochemistry 2021, 138, 107701. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Highly sensitive and strongly fluorescent gum tragacanth based superabsorbent hydrogel as a new biosensor for glucose optical detection. J. Mater. Chem. C 2020, 8, 4148–4156. [Google Scholar] [CrossRef]
- Qasemi, S.; Ghaemy, M. Novel superabsorbent biosensor nanohydrogel based on gum tragacanth polysaccharide for optical detection of glucose. Int. J. Biol. Macromol. 2020, 151, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Bhagel, D.; Mishra, P.; Prasad, D.; Kohli, E. Post-synthetic modification of graphene quantum dots bestows enhanced biosensing and antibiofilm ability: Efficiency facet. RSC Adv. 2022, 12, 12310–12320. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.; Zhang, X.; Zhou, Z.; Qian, J.; Liu, Q.; Chen, S.; Zhang, Y.; Wang, K. Three-dimensional nitrogen-doped graphene porous hydrogel fabricated biosensing platform with enhanced photoelectrochemical performance. Sens. Actuators B Chem. 2017, 250, 476–483. [Google Scholar] [CrossRef]
- Li, H.; Su, C.; Liu, N.; Lv, T.; Yang, C.; Lu, Q.; Sun, C.; Yan, X. Carbon Dot-Anchored Cobalt Oxyhydroxide Composite-Based Hydrogel Sensor for On-Site Monitoring of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2022, 14, 53340–53347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H. Synthesis and characterization of carboxymethyl cellulose/layered double hydroxide nanocomposites. J. Nanoparticle Res. 2013, 15, 1563. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin loaded carboxymethyl cellulose/graphene quantum dot nanocomposite hydrogel films as a potential anticancer drug delivery system. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Singh, S.; Mishra, A.; Kumari, R.; Sinha, K.K.; Singh, M.K.; Das, P. Carbon dots assisted formation of DNA hydrogel for sustained release of drug. Carbon 2017, 114, 169–176. [Google Scholar] [CrossRef]
- Rakhshaei, R.; Namazi, H.; Hamishehkar, H.; Rahimi, M. Graphene quantum dot cross-linked carboxymethyl cellulose nanocomposite hydrogel for pH-sensitive oral anticancer drug delivery with potential bioimaging properties. Int. J. Biol. Macromol. 2020, 150, 1121–1129. [Google Scholar] [CrossRef] [PubMed]








| Fabrication Method | Classification | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Sol–gel method | Chemical and physical | High purity, precise control over nanoparticle size and distribution, low cost | Long synthesis time, difficult to scale up | [71] |
| Emulsion polymerization | Surfactant-mediated and Surfactant-free | High yield, fast synthesis time, versatile in terms of polymerization conditions | Poor control over particle size, low stability, high surfactant content | [72,73] |
| Electrospinning | Coaxial and single jet | Precise control over fiber morphology, good mechanical properties, versatile in terms of polymer selection | Limited to fiber formation, limited control over nanoparticle distribution | [74] |
| In situ polymerization | Radical-triggered and ionic polymerization | Easy to scale up, good control over particle size and distribution | Limited control over particle morphology, may require toxic solvents | [75,76] |
| Layer-by-layer assembly | Polyelectrolyte-based and hybrid | Versatile, precise control over nanoparticle distribution and thickness, easy to incorporate functional groups | Time-consuming, requires careful optimization of conditions | [77,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandla, K.; Kumar, K.P.; Rajasulochana, P.; Charde, M.S.; Rana, R.; Singh, L.P.; Haque, M.A.; Bakshi, V.; Siddiqui, F.A.; Khan, S.L.; et al. Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications. Gels 2023, 9, 669. https://doi.org/10.3390/gels9080669
Gandla K, Kumar KP, Rajasulochana P, Charde MS, Rana R, Singh LP, Haque MA, Bakshi V, Siddiqui FA, Khan SL, et al. Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications. Gels. 2023; 9(8):669. https://doi.org/10.3390/gels9080669
Chicago/Turabian StyleGandla, Kumaraswamy, K. Praveen Kumar, P. Rajasulochana, Manoj Shrawan Charde, Ritesh Rana, Laliteshwar Pratap Singh, M. Akiful Haque, Vasudha Bakshi, Falak A. Siddiqui, Sharuk L. Khan, and et al. 2023. "Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications" Gels 9, no. 8: 669. https://doi.org/10.3390/gels9080669
APA StyleGandla, K., Kumar, K. P., Rajasulochana, P., Charde, M. S., Rana, R., Singh, L. P., Haque, M. A., Bakshi, V., Siddiqui, F. A., Khan, S. L., & Ganguly, S. (2023). Fluorescent-Nanoparticle-Impregnated Nanocomposite Polymeric Gels for Biosensing and Drug Delivery Applications. Gels, 9(8), 669. https://doi.org/10.3390/gels9080669