Comprehensive Study on Carbon-Coated Silver for Improved Tribo-Electrical and Wetting Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Techniques
3. Results and Discussion
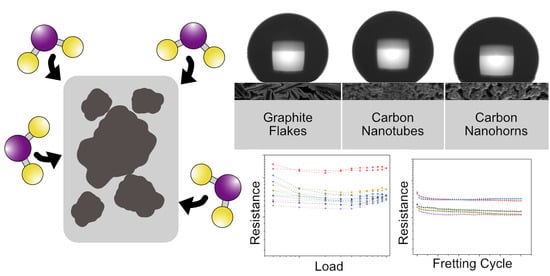
3.1. Load-Dependent ECR
3.2. ECR Evolution during Fretting Test
3.3. Wetting Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Outka, D.A.; Madix, R.J.; Fisher, G.B.; DiMaggio, C. Oxidation of Sulfur Dioxide on Silver (110): Vibrational Study of the Structure of Intermediate Complexes Formed. J. Phys. Chem. 1986, 90, 4051–4057. [Google Scholar] [CrossRef]
- Bauer, R. Sulfide Corrosion of Silver Contacts during Satellite Storage. J. Spacecr. Rocket. 1988, 25, 439–440. [Google Scholar] [CrossRef]
- Lilienfeld, S.; White, C.E. A Study of the Reaction between Hydrogen Sulfide and Silver. J. Am. Chem. Soc. 1930, 52, 885–892. [Google Scholar] [CrossRef]
- Russ, G. Electrical Characteristics of Contacts Contaminated with Silver Sulfide Film. IEEE Trans. Parts Mater. Packag. 1970, 6, 129–137. [Google Scholar] [CrossRef]
- Hindin, B.S. Silver Sulfide Corrosion Control Using Corrosion Prevention Compounds. In Proceedings of the CORROSION 2006, San Diego, CA, USA, 12–16 March 2006; p. NACE-06264. [Google Scholar]
- Denaburg, C.R. Corrosion in Aerospace Electrical/Electronic Components in the Early Years of the Apollo Program, Kennedy Space Center, Florida. In Proceedings of the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000; p. NACE-00718. [Google Scholar]
- Schmitt, W.; Franz, S.; Heber, J.; Lutz, O.; Behrens, V. Formation of Silver Sulfide Layers and Their Influence on the Electrical Characteristics of Contacts in the Field of Information Technology. In Proceedings of the ICEC, Innsbruck, Austria, 19–22 August 2008; pp. 489–494. [Google Scholar]
- Wagner, C. Investigations on Silver Sulfide. J. Chem. Phys. 1953, 21, 1819–1827. [Google Scholar] [CrossRef]
- Hebb, M.H. Electrical Conductivity of Silver Sulfide. J. Chem. Phys. 1952, 20, 185–190. [Google Scholar] [CrossRef]
- Sahraoui, K.; Benramdane, N.; Khadraoui, M.; Miloua, R.; Mathieu, C. Characterization of Silver Sulphide Thin Films Prepared by Spray Pyrolysis Using a New Precursor Silver Chloride. Sens. Transducers 2014, 27, 319–325. [Google Scholar]
- Zhang, S.; Osterman, M.; Shrivastava, A.; Kang, R.; Pecht, M.G. The Influence of H2S Exposure on Immersion-Silver-Finished PCBs under Mixed-Flow Gas Testing. IEEE Trans. Device Mater. Reliab. 2010, 10, 71–81. [Google Scholar] [CrossRef]
- Abbott, W.H. The Development and Performance Characteristics of Mixed Flowing Gas Test Environment. IEEE Trans. Compon. Hybrids Manuf. Technol. 1988, 11, 22–35. [Google Scholar] [CrossRef]
- Ying, T. An Advanced Anti-Tarnish Process for Silver Coins and Silverware—Monomolecular Octadecanethiol Protective Film. Tribol. Trans. 2021, 64, 341–349. [Google Scholar] [CrossRef]
- Liang, C.; Yang, C.; Huang, N. Tarnish Protection of Silver by Octadecanethiol Self-Assembled Monolayers Prepared in Aqueous Micellar Solution. Surf. Coat. Technol. 2009, 203, 1034–1044. [Google Scholar] [CrossRef]
- Liang, C.H.; Yang, C.J.; Huang, N.B.; Wu, B. Comparison of Four Antitarnishing Self-Assembled Monolayers on Silver Coin. Surf. Eng. 2011, 27, 199–204. [Google Scholar] [CrossRef]
- Phillips, A.C.; Cowley, A. Octadecanthiol for Tarnish-Resistant Silver Coatings. In Proceedings of the Modern Technologies in Space- and Ground-Based Telescopes and Instrumentation II, Amsterdam, The Netherlands, 1–6 July 2012; Navarro, R., Cunningham, C.R., Prieto, E., Eds.; Volume 8450, p. 84503W. [Google Scholar]
- Planes, E.; Flandin, L.; Alberola, N. Polymer Composites Bipolar Plates for PEMFCs. Energy Procedia 2012, 20, 311–323. [Google Scholar] [CrossRef]
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998; ISBN 978-1-86094-093-4. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R. Physics of Carbon Nanotubes. Carbon 1995, 33, 883–891. [Google Scholar] [CrossRef]
- Popov, V. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Carbon Nanotubes. Annu. Rev. Mater. Sci. 1994, 24, 235–264. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Zainal, Z.; Yusof, N. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-G.; Hwang, Y.-J.; Choi, Y.-M.; Lee, J.-K.; Choi, C.; Oh, J.-M. A Study on the Tribological Characteristics of Graphite Nano Lubricants. Int. J. Precis. Eng. Manuf. 2009, 10, 85–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Simionesie, D.; Schaschke, C. Graphite and Hybrid Nanomaterials as Lubricant Additives. Lubricants 2014, 2, 44–65. [Google Scholar] [CrossRef]
- Cornelio, J.A.C.; Cuervo, P.A.; Hoyos-Palacio, L.M.; Lara-Romero, J.; Toro, A. Tribological Properties of Carbon Nanotubes as Lubricant Additive in Oil and Water for a Wheel–Rail System. J. Mater. Res. Technol. 2016, 5, 68–76. [Google Scholar] [CrossRef]
- Dassenoy, F.; Joly-Pottuz, L.; Martin, J.M.; Mieno, T. Carbon Nanotubes as Advanced Lubricant Additives. In Carbon Nanotubes; Popov, V.N., Lambin, P., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2006; pp. 237–238. [Google Scholar]
- Kałużny, J.; Merkisz-Guranowska, A.; Giersig, M.; Kempa, K. Lubricating Performance of Carbon Nanotubes in Internal Combustion Engines—Engine Test Results for CNT Enriched Oil. Int. J. Automot. Technol. 2017, 18, 1047–1059. [Google Scholar] [CrossRef]
- Maharaj, D.; Bhushan, B.; Iijima, S. Effect of Carbon Nanohorns on Nanofriction and Wear Reduction in Dry and Liquid Environments. J. Colloid. Interface Sci. 2013, 400, 147–160. [Google Scholar] [CrossRef]
- Sethi, S.; Dhinojwala, A. Superhydrophobic Conductive Carbon Nanotube Coatings for Steel. Langmuir 2009, 25, 4311–4313. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.; Roy, R.; Younis, H.; AlNahyan, M.; Younes, H. Carbon Nanomaterial-Based Lubricants: Review of Recent Developments. Lubricants 2022, 10, 281. [Google Scholar] [CrossRef]
- De Nicola, F.; Castrucci, P.; Scarselli, M.; Nanni, F.; Cacciotti, I.; Crescenzi, M. De Super-Hydrophobic Multi-Walled Carbon Nanotube Coatings for Stainless Steel. Nanotechnology 2015, 26, 145701. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J. Superlubricity of Carbon Nanostructures. Carbon 2020, 158, 1–23. [Google Scholar] [CrossRef]
- Loyd, A.; Hemond, J.; Martens, R. A Preliminary Investigation of Graphite, Graphene and Carbon Nanotubes (CNT’s) as Solid State Lubricants. In Proceedings of the 2011 IEEE 57th Holm Conference on Electrical Contacts (Holm), Minneapolis, MN, USA, 11–14 September 2011; IEEE: Piscataway, NJ, USA, 2011; pp. 1–9. [Google Scholar]
- Reinert, L.; Varenberg, M.; Mücklich, F.; Suárez, S. Dry Friction and Wear of Self-Lubricating Carbon-Nanotube-Containing Surfaces. Wear 2018, 406–407, 33–42. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Characterization and Electrical Analysis of Carbon-Based Solid Lubricant Coatings. Carbon Trends 2022, 7, 100156. [Google Scholar] [CrossRef]
- Alderete, B.; MacLucas, T.; Espin, D.; Brühl, S.P.; Mücklich, F.; Suarez, S. Near Superhydrophobic Carbon Nanotube Coatings Obtained via Electrophoretic Deposition on Low-Alloy Steels. Adv. Eng. Mater. 2021, 23, 2001448. [Google Scholar] [CrossRef]
- Alderete, B.; Lößlein, S.M.; Bucio Tejeda, D.; Mücklich, F.; Suarez, S. Feasibility of Carbon Nanoparticle Coatings as Protective Barriers for Copper—Wetting Assessment. Langmuir 2022, 38, 15209–15219. [Google Scholar] [CrossRef]
- Huo, Y.; Fu, S.-W.; Chen, Y.-L.; Lee, C.C. A Reaction Study of Sulfur Vapor with Silver and Silver–Indium Solid Solution as a Tarnishing Test Method. J. Mater. Sci. Mater. Electron. 2016, 27, 10382–10392. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion Analysis of Carbon Nanotubes, Carbon Onions, and Nanodiamonds for Their Application as Reinforcement Phase in Nickel Metal Matrix Composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef]
- Philadelphia Museum of Art Finishing Techniques in Metalwork—Hands-Free Silver “Polishing”. Available online: https://www.philamuseum.org/booklets/7_44_85_1.html?page=2 (accessed on 18 January 2021).
- Alderete, B.; Mücklich, F.; Suarez, S. Tarnishing (Ag2S) Layer on Silver-Plated Electrical Contacts: Its Influence on Electrical Contact Resistance. IEEE Trans. Compon. Packag. Manuf. Technol. 2023, 13, 45–58. [Google Scholar] [CrossRef]
- Alderete, B.; Mücklich, F.; Suarez, S. Wear Reduction via CNT Coatings in Electrical Contacts Subjected to Fretting. Tribol. Lett. 2023, 71, 54. [Google Scholar] [CrossRef]
- Alderete, B.; Suarez, S.; Tejeda, D.B.; Mücklich, F. Fretting and Electrical Contact Resistance Characteristics of Carbon Nanoparticle-Coated Cu Electrical Contacts. In Proceedings of the 2022 IEEE 67th Holm Conference on Electrical Contacts (HLM), Tampa, FL, USA, 23–26 October 2022; IEEE: Piscataway, NJ, USA, 2022; pp. 1–8. [Google Scholar]
- Thomas, B.J.C.; Boccaccini, A.R.; Shaffer, M.S.P. Multi-Walled Carbon Nanotube Coatings Using Electrophoretic Deposition (EPD). J. Am. Ceram. Soc. 2005, 88, 980–982. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Jane Minay, E.; Shaffer, M.S.P. Electrophoretic Deposition of Carbon Nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Alderete, B.; Nayak, U.P.; Mücklich, F.; Suarez, S. Influence of Topography on Electrical Contact Resistance of Copper-Based Materials. Surf. Topogr. 2023, 11, 025027. [Google Scholar] [CrossRef]
- Vafaei, S.; Podowski, M.Z. Theoretical Analysis on the Effect of Liquid Droplet Geometry on Contact Angle. Nucl. Eng. Des. 2005, 235, 1293–1301. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact Angle Measurement on Porous Substrates: Effect of Liquid Absorption and Drop Size. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Alderete, B.; Puyol, R.; Slawik, S.; Espin, E.; Mücklich, F.; Suarez, S. Multipurpose Setup Used to Characterize Tribo-Electrical Properties of Electrical Contact Materials. MethodsX 2021, 8, 101498. [Google Scholar] [CrossRef] [PubMed]
- Bock, E.M. Low-Level Contact Resistance Characterization. AMP J. Technol. 1993, 3, 64–68. [Google Scholar]
- Johnson, K.L. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1987; ISBN 0521347963. [Google Scholar]
- Callister, W.D., Jr. Materials Science and Engineering: An Introduction, 7th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 94, ISBN 9780470054888. [Google Scholar]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of Humidity on Gas Sensing Performance of Carbon Nanotube Gas Sensors Operated at Room Temperature. IEEE Sens. J. 2021, 21, 5763–5770. [Google Scholar] [CrossRef]
- Tsai, J.T.H.; Lu, C.-C.; Li, J.G. Fabrication of Humidity Sensors by Multi-Walled Carbon Nanotubes. J. Exp. Nanosci. 2010, 5, 302–309. [Google Scholar] [CrossRef]
- Lee, Y.; Yoon, J.; Kim, Y.; Kim, D.M.; Kim, D.H.; Choi, S.-J. Humidity Effects According to the Type of Carbon Nanotubes. IEEE Access 2021, 9, 6810–6816. [Google Scholar] [CrossRef]
- Ling, Y.; Gu, G.; Liu, R.; Lu, X.; Kayastha, V.; Jones, C.S.; Shih, W.-S.; Janzen, D.C. Investigation of the Humidity-Dependent Conductance of Single-Walled Carbon Nanotube Networks. J. Appl. Phys. 2013, 113, 024312. [Google Scholar] [CrossRef]
- Holm, R. Electric Contacts, 4th ed.; Springer: Berlin/Heidelberg, Germany, 1967; ISBN 978-3-642-05708-3. [Google Scholar]
- Endres, H. Praxishandbuch Steckverbinder, 2nd ed.; Vogel COmmunications Group: Würzburg, Germany, 2021; ISBN 978-3-8343-3501-2. [Google Scholar]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Slade, P.G. Electrical Contacts, 2nd ed.; Slade, P.G., Ed.; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781315216829. [Google Scholar]
- Merstallinger, A.; Sales, M.; Semerad, E.; Dunn, B.D. Assessment of Cold Welding between Separable Contact Surfaces Due to Impact and Fretting under Vacuum. ESA Sci. Tech. Memo. 2009, 279, 57. [Google Scholar]
- Haddon, R.C. Chemistry of the Fullerenes: The Manifestation of Strain in a Class of Continuous Aromatic Molecules. Science 1993, 261, 1545–1550. [Google Scholar] [CrossRef]
- Korczeniewski, E.; Zięba, M.; Zięba, W.; Kolanowska, A.; Bolibok, P.; Kowalczyk, P.; Wiertel-Pochopień, A.; Zawała, J.; Boncel, S.; Terzyk, A.P. Electrophoretic Deposition of Layer-by-Layer Unsheathed Carbon Nanotubes—A Step Towards Steerable Surface Roughness and Wettability. Materials 2020, 13, 595. [Google Scholar] [CrossRef]
- Hilding, J.; Grulke, E.A.; George Zhang, Z.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]






| Surface | Roughness/µm |
|---|---|
| Reference | 0.26 ± 0.03 |
| Tarnished | 0.40 ± 0.04 |
| GF10 | 1.40 ± 0.12 |
| CNT5 | 0.91 ± 0.16 |
| CNT10 | 0.62 ± 0.02 |
| CNH10 | 0.51 ± 0.01 |
| Coating | Thickness/µm |
|---|---|
| GF10 | 0.34 ± 0.10 |
| CNT5 | 0.14 ± 0.04 |
| CNT10 | 0.41 ± 0.05 |
| CNH10 | 0.32 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderete, B.; Mücklich, F.; Suarez, S. Comprehensive Study on Carbon-Coated Silver for Improved Tribo-Electrical and Wetting Performance. C 2024, 10, 16. https://doi.org/10.3390/c10010016
Alderete B, Mücklich F, Suarez S. Comprehensive Study on Carbon-Coated Silver for Improved Tribo-Electrical and Wetting Performance. C. 2024; 10(1):16. https://doi.org/10.3390/c10010016
Chicago/Turabian StyleAlderete, Bruno, Frank Mücklich, and Sebastian Suarez. 2024. "Comprehensive Study on Carbon-Coated Silver for Improved Tribo-Electrical and Wetting Performance" C 10, no. 1: 16. https://doi.org/10.3390/c10010016
APA StyleAlderete, B., Mücklich, F., & Suarez, S. (2024). Comprehensive Study on Carbon-Coated Silver for Improved Tribo-Electrical and Wetting Performance. C, 10(1), 16. https://doi.org/10.3390/c10010016