Application of L-FDM Technology to the Printing of Tablets That Release Active Substances—Preliminary Research
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
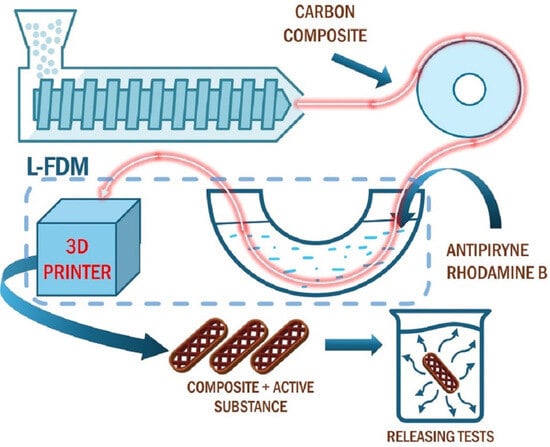
2.2.1. Extrusion of Filament with 5% Activated Carbon Content
2.2.2. Printing of Tiles with Different Top and Infill Patterns
2.2.3. L-FDM Printing Process
2.3. Analyses
3. Results and Discussion
3.1. WCAs of Tiles with Different Infill Patterns
3.2. Microscopic Observation
3.3. Release Experiments
3.4. Surface Area Characterization Using Low-Temperature Nitrogen Adsorption Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kadry, H.; Al-Hilal, T.A.; Keshavarz, A.; Alam, F.; Xu, C.; Joy, A.; Ahsan, F. Multi-Purposable Filaments of HPMC for 3D Printing of Medications with Tailored Drug Release and Timed-Absorption. Int. J. Pharm. 2018, 544, 285–296. [Google Scholar] [CrossRef]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.N.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2021, 56, 910–928. [Google Scholar] [CrossRef]
- Amekyeh, H.; Tarlochan, F.; Billa, N. Practicality of 3D Printed Personalized Medicines in Therapeutics. Front. Pharmacol. 2021, 12, 646836. [Google Scholar] [CrossRef]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Haris, M.S.; Azlan, N.H.M.; Taher, M.; Rus, S.M.; Chatterjee, B. 3D-Printed Drugs: A Fabrication of Pharmaceuticals towards Personalized Medicine. Indian J. Pharm. Educ. Res. 2020, 54, s411–s422. [Google Scholar] [CrossRef]
- Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. [Google Scholar] [CrossRef]
- Agrawal, A.; Gupta, A.K. 3D printing technology in pharmaceuticals and biomedical: A review. J. Drug Deliv. Ther. 2019, 9, 1–4. [Google Scholar]
- Kantaros, A. 3D Printing in Regenerative Medicine: Technologies and Resources Utilized. Int. J. Mol. Sci. 2022, 23, 14621. [Google Scholar] [CrossRef]
- Melčová, V.; Svoradová, K.; Menčík, P.; Kontárová, S.; Rampichová, M.; Hedvičáková, V.; Sovková, V.; Přikryl, R.; Vojtová, L. FDM 3D Printed Composites for Bone Tissue Engineering Based on Plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends. Polymers 2020, 12, 2806. [Google Scholar] [CrossRef] [PubMed]
- Afsana; Jain, V.; Haider, N.; Jain, K. 3D Printing in Personalized Drug Delivery. Curr. Pharm. Des. 2019, 24, 5062–5071. [Google Scholar] [CrossRef] [PubMed]
- Kantaros, A.; Ganetsos, T.; Petrescu, F.I.T. Transforming Object Design and Creation: Biomaterials and Contemporary Manufacturing Leading the Way. Biomimetics 2024, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Deshmane, S.V.; Kendre, P.N.; Mahajan, H.S.; Jain, S.P. Stereolithography 3D Printing Technology in Pharmaceuticals: A Review. Drug Dev. Ind. Pharm. 2021, 47, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of Release Modifiers to Modulate Drug Release from Fused Deposition Modelling (FDM) 3D Printed Tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, H.; Huang, L.; Zhang, M.; Fan, W.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.G.; Branford-White, C.; Yang, Y.C.; Zhu, L.M.; Welbeck, E.W.; Yang, X.L. A novel fast disintegrating tablet fabricated by three-dimensional printing. Drug Dev. Ind. Pharm. 2009, 35, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Gui, W.S.; Verani. Building porous biopolymeric microstructures for controlled drug delivery devices using selective laser sintering. Int. J. Adv. Manuf. Technol. 2006, 31, 483–489. [Google Scholar] [CrossRef]
- Sharma, P.K.; Choudhury, D.; Yadav, V.; Murty, U.S.N.; Banerjee, S. 3D printing of nanocomposite pills through desktop vat photopolymerization (stereolithography) for drug delivery reasons. 3D Print. Med. 2022, 8, 3. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Cui, M.; Zhu, Z.; Chen, K.; Wen, H.; Jia, D.; Hou, J.; Xu, W.; Yang, X.; et al. Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing. Int. J. Pharm. 2018, 535, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kozakiewicz-Latała, M.; Junak, A.; Złocińska, A.; Pudło, W.; Prusik, K.; Szymczyk-Ziółkowska, P.; Karolewicz, B.; Nartowski, K.P. Adjusting the melting point of an Active Pharmaceutical Ingredient (API) via cocrystal formation enables processing of high melting drugs via combined hot melt and materials extrusion (HME and ME). Addit. Manuf. 2022, 60, 103196. [Google Scholar] [CrossRef]
- Buyukgoz, G.G.; Soffer, D.; Defendre, J.; Pizzano, G.M.; Davé, R.N. Exploring tablet design options for tailoring drug release and dose via fused deposition modeling (FDM) 3D printing. Int. J. Pharm. 2020, 591, 119987. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Buanz, A.B.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Briatico-Vangosa, F.; Uboldi, M.; Parietti, F.; Turchi, M.; von Zeppelin, D.; Maroni, A.; Zema, L.; Gazzaniga, A.; Zidan, A. Quality considerations on the pharmaceutical applications of fused deposition modeling 3D printing. Int. J. Pharm. 2021, 592, 119901. [Google Scholar] [CrossRef]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef]
- Kantaros, A.; Ganetsos, T. From Static to Dynamic: Smart Materials Pioneering Additive Manufacturing in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 15748. [Google Scholar] [CrossRef]
- Park, S.; Kim, B. A study on NO removal of activated carbon fibers with deposited silver nanoparticles. J. Colloid Interface Sci. 2005, 282, 124–127. [Google Scholar] [CrossRef]
- Hung, M.; Yuan, S.; Chang, S.I.; Liao, J.; Ko, T.; Cheng, C. Evaluation of active carbon fibers used in cell biocompatibility and rat cystitis treatment. Carbon 2014, 68, 628–637. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Activated Carbon; The Royal Society of Chemistry: London, UK, 2023. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in wound dressings. Clin. Dermatol. 2006, 25, 33–38. [Google Scholar] [CrossRef]
- Lewoyehu, M. Comprehensive review on synthesis and application of activated carbon from agricultural residues for the remediation of venomous pollutants in wastewater. J. Anal. Appl. Pyrolysis 2021, 159, 105279. [Google Scholar] [CrossRef]
- Yadavalli, T.; Ames, J.; Agelidis, A.; Suryawanshi, R.; Jaishankar, D.; Hopkins, J.; Thakkar, N.; Koujah, L.; Shukla, D. Drug-encapsulated carbon (DECON): A novel platform for enhanced drug delivery. Sci Adv. 2019, 5, eaax0780. [Google Scholar] [CrossRef] [PubMed]
- Olivier, F.; Bonnamy, S.; Rochet, N.; Drouet, C. Activated Carbon Fiber Cloth/Biomimetic Apatite: A Dual Drug Delivery System. Int. J. Mol. Sci. 2021, 22, 12247. [Google Scholar] [CrossRef]
- Nazarkina, Z.K.; Stepanova, A.O.; Chelobanov, B.P.; Kvon, R.I.; Simonov, P.A.; Karpenko, A.A.; Laktionov, P.P. Activated Carbon-Enriched Electrospun-Produced Scaffolds for Drug Delivery/Release in Biological Systems. Int. J. Mol. Sci. 2022, 24, 6713. [Google Scholar] [CrossRef] [PubMed]
- Sztorch, B.; Brząkalski, D.; Pakuła, D.; Frydrych, M.; Špitalský, Z.; Przekop, R.E. Natural and Synthetic Polymer Fillers for Applications in 3D Printing—FDM Technology Area. Solids 2022, 3, 508–548. [Google Scholar] [CrossRef]
- Balou, S.; Ahmed, I.; Priye, A. From Waste to Filament: Development of Biomass-Derived Activated Carbon-Reinforced PETG Composites for Sustainable 3D Printing. ACS Sustain. Chem. Eng. 2023, 11, 12667–12676. [Google Scholar] [CrossRef]
- Przekop, R.E.; Kujawa, M.; Pawlak, W.; Dobrosielska, M.; Sztorch, B.; Wieleba, W. Graphite Modified Polylactide (PLA) for 3D Printed (FDM/FFF) Sliding Elements. Polymers 2020, 12, 1250. [Google Scholar] [CrossRef]
- Yang, L.; Li, S.; Zhou, X.; Liu, J.; Li, Y.; Yang, M.; Yuan, Q.; Zhang, W. Effects of carbon nanotube on the thermal, mechanical, and electrical properties of PLA/CNT printed parts in the FDM process. Synth. Met. 2019, 253, 122–130. [Google Scholar] [CrossRef]
- Przekop, R.E.; Gabriel, E.; Pakuła, D.; Sztorch, B. Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements. Appl. Sci. 2023, 13, 7393. [Google Scholar] [CrossRef]
- Przekop, R.E.; Gabriel, E.; Pakuła, D.; Sztorch, B. Liquid to Fused Deposition Modeling (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology—Mechanical and Functional Properties. Appl. Sci. 2023, 13, 8462. [Google Scholar] [CrossRef]
- Bettini, R.; Catellani, P.L.; Santi, P.; Massimo, G.; Peppas, N.A.; Colombo, P. Translocation of drug particles in HPMC matrix gel layer: Effect of drug solubility and influence on release rate. J. Control. Release 2001, 70, 383–391. [Google Scholar] [CrossRef]
- Kamaludin, N.H.I.; Ismail, H.; Rusli, A.; Ting, S.S. Thermal behavior and water absorption kinetics of polylactic acid/chitosan biocomposites. Iran Polym. J. 2021, 30, 135–147. [Google Scholar] [CrossRef]
- Zghal, S.; Jedidi, I.; Cretin, M.; Cerneaux, S.; Abdelmouleh, M. Adsorptive Removal of Rhodamine B Dye Using Carbon Graphite/CNT Composites as Adsorbents: Kinetics, Isotherms and Thermodynamic Study. Materials 2023, 16, 1015. [Google Scholar] [CrossRef]











| Parameter | Value | |
|---|---|---|
| Slicer | Prusa Slicer | |
| Layer height [mm] | 0.2 | |
| Printing temperature [°C] | 210 | |
| Bed temperature [°C] | 60 | |
| Number of shells | 2 | |
| Printing speed [mm/s] | 40 | |
| First layer speed [mm/s] | 10 | |
| Combine infill every [layer] | 1 | |
| Tiles with different top infill pattern | Tiles with different infill pattern | |
| Top/bottom solid layers | 2/2 | 0/2 |
| Infill type | monotonic | Cubic/gyroid/stars/Hilbert curve |
| Top infill type * | Monotonic/Archimedean chords/Hilbert curve/octagram spiral | - |
| Infill % | 100 | 99 |
| Parameter | Value |
|---|---|
| Slicer | Prusa Slicer |
| Layer height [mm] | 0.2 |
| Printing temperature [°C] | 210 |
| Bed temperature [°C] | 60 |
| Number of shells | 2 |
| Top/bottom solid layers | 0 |
| Retraction length [mm]/speed [mm/s] | 6/60 |
| Infill type | Rectlinear |
| Infill % | 20 |
| Combine infill every [layer] | 1 |
| Printing speed [mm/s] | 10/20/40 |
| First layer speed [mm/s] | 10 |
| Top Infill | Inner Infill | ||
|---|---|---|---|
| Monotonic | 92.66 ± 6.13 | Cubic | 96.18 ± 5.56 |
| Archimedean chord | 73.48 ± 6.56 | Gyroid | 73.88 ± 1.99 |
| Hilbert curve | 79.46 ± 6.94 | Stars | 81.02 ± 5.00 |
| Octogram spiral | 75.56 ± 6.88 | Hilbert curve | 81.48 ± 6.78 |
| Sample Name | BET Surface Area [m2/g] | Pore Volume [cm3/g] | Average Pore Diameter [nm] |
|---|---|---|---|
| PLA granulate | 0 | 0.006 | 3.317 |
| CA powder | 779 | 0.202 | 3.938 |
| CA 5% tablet | 40 | 0.005 | 3.141 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabriel, E.; Olejnik, A.; Sztorch, B.; Frydrych, M.; Czerwińska, O.; Pietrzak, R.; Przekop, R.E. Application of L-FDM Technology to the Printing of Tablets That Release Active Substances—Preliminary Research. C 2024, 10, 23. https://doi.org/10.3390/c10010023
Gabriel E, Olejnik A, Sztorch B, Frydrych M, Czerwińska O, Pietrzak R, Przekop RE. Application of L-FDM Technology to the Printing of Tablets That Release Active Substances—Preliminary Research. C. 2024; 10(1):23. https://doi.org/10.3390/c10010023
Chicago/Turabian StyleGabriel, Ewa, Anna Olejnik, Bogna Sztorch, Miłosz Frydrych, Olga Czerwińska, Robert Pietrzak, and Robert E. Przekop. 2024. "Application of L-FDM Technology to the Printing of Tablets That Release Active Substances—Preliminary Research" C 10, no. 1: 23. https://doi.org/10.3390/c10010023
APA StyleGabriel, E., Olejnik, A., Sztorch, B., Frydrych, M., Czerwińska, O., Pietrzak, R., & Przekop, R. E. (2024). Application of L-FDM Technology to the Printing of Tablets That Release Active Substances—Preliminary Research. C, 10(1), 23. https://doi.org/10.3390/c10010023