Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
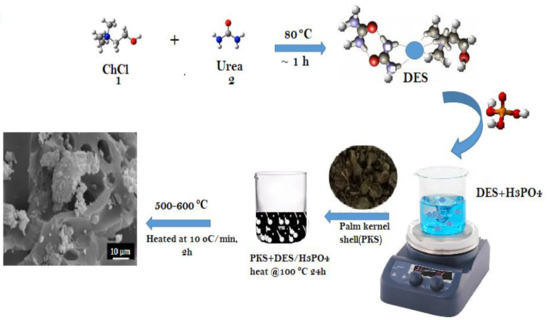
2.2. Synthesis of Choline Chloride–Urea Deep Eutectic Solvent
2.3. Preparation of Activated Carbon
2.4. Characterization of Adsorbents
Specific Surface Area and Pore-Distribution
2.5. Functional Groups Determination
2.6. Morphological Characterization
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy
3.2. Surface Morphology
3.3. BET Surface Area and Pore Characteristics
3.4. Adsorption of Lead
4. Conclusions
Funding
Conflicts of Interest
References
- Qin, C.; Liu, B.; Huang, L.; Liang, C.; Gao, C.; Yao, S. Adsorptive removal of adsorbable organic halogens by activated carbon. R. Soc. Open Sci. 2018, 5, 181507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cukierman, A.L. Development and environmental applications of activated carbon cloths. ISRN Chem. Eng. 2013. [Google Scholar] [CrossRef]
- Fałtynowicz, H.; Kaczmarczyk, J.; Kułażyński, M. Preparation and characterization of activated carbons from biomass material–giant knotweed (Reynoutria sachalinensis). Open Chem. 2015, 13, 1150–1156. [Google Scholar]
- Peng, Z.; Guo, Z.; Chu, W.; Wei, M. Facile synthesis of high-surface-area activated carbon from coal for supercapacitors and high CO2 sorption. RSC Adv. 2016, 6, 42019–42028. [Google Scholar] [CrossRef]
- Miriyala, N.; Ouyang, D.; Perrie, Y.; Lowry, D.; Kirby, D.J. Activated carbon as a carrier for amorphous drug delivery: Effect of drug characteristics and carrier wettability. Eur. J. Pharm. Biopharm. 2017, 115, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Gioia, M.; Nardi, M.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. Biorenewable deep eutectic solvent for selective and scalable conversion of furfural into cyclopentenone derivatives. Molecules 2018, 23, 1891. [Google Scholar] [CrossRef] [PubMed]
- AlOmar, M.K.; Alsaadi, M.A.; Jassam, T.M.; Akib, S.; Hashim, M.A. Novel deep eutectic solvent-functionalized carbon nanotubes adsorbent for mercury removal from water. J. Colloid Interface Sci. 2017, 497, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (dess) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Zhang, J.; Liu, R. Synthesis of spherical Fe3O4 magnetic nanoparticles by co-precipitation in choline chloride/urea deep eutectic solvent. Mater. Lett. 2013, 112, 177–179. [Google Scholar] [CrossRef]
- Li, X.; Choi, J.; Ahn, W.S.; Row, K.H. Preparation and application of porous materials based on deep eutectic solvents. Crit. Rev. Anal. Chem. 2018, 48, 73–85. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of Fe3O4 magnetic nanoparticles as efficient heterogeneous Fenton-like catalysts for degradation of organic pollutants with H2O2. J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Patiño, J.; Gutiérrez, M.C.; Carriazo, D.; Ania, C.O.; Parra, J.B.; Ferrer, M.L.; del Monte, F. Deep eutectic assisted synthesis of carbon adsorbents highly suitable for low- pressure separation of CO2/CH4 gas mixtures. Energy Environ. Sci. 2012, 5, 8699–8707. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Fabrication of water-compatible molecularly imprinted resin in a hydrophilic deep eutectic solvent for the determination and purification of quinolones in wastewaters. Polymers 2019, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.A.; Dolla, T.H.; Pruessner, K.; Ndungu, P. Synthesis and characterization of deep eutectic solvent functionalized CNT/ZnCo2O4 nanostructure: Kinetics, isotherm and regenerative studies on Eosin y adsorption. J. Environ. Chem. Eng. 2019, 7, 102877. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; El-Shafie, A.; Hin, L.S.; Mohd, N.S.B.; Aljumaily, M.M.; Ibraim, S.; AlSaadi, M.A.A. Clean approach for functionalized carbon nanotubes by deep eutectic solvents and their performance in the adsorption of methyl orange from aqueous solution. J. Environ. Manag. 2019, 235, 521–534. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Pan, Q.; Wang, Y.; Ding, X.; Xu, K.; Li, N.; Wen, Q.W. Magnetic graphene oxide modified with choline chloride-based deep eutectic solvent for the solid-phase extraction of protein. Anal. Chim. Acta 2015, 877, 87790–87799. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.; Row, K.H. Graphene and graphene oxide modified by deep eutectic solvents and ionic liquids supported on silica as adsorbents for solid-phase extraction. Bull. Korean Chem. Soc. 2017, 38, 251–257. [Google Scholar] [CrossRef]
- Carriazo, D.; Gutiérrez, M.C.; Picó, F.; Rojo, J.M.; Fierro, J.L.; Ferrer, M.L.; del Monte, F. Phosphate-functionalized carbon monoliths from deep eutectic solvents and their use as monolithic electrodes in supercapacitors. ChemSusChem 2012, 5, 1405–1409. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, R.; Li, G.; Hu, C. The role of H3PO4 in the preparation of activated carbon from NaOH-treated rice husk residue. Rsc Adv. 2015, 5, 32626–32636. [Google Scholar] [CrossRef]
- Yadav, A.; Pandey, S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K. J. Chem. Eng. Data 2014, 59, 2221–2229. [Google Scholar] [CrossRef]
- Kalderis, D.; Bethanis, S.; Paraskeva, P.; Diamadopoulos, E. Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times. Bioresour. Technol. 2008, 99, 6809–6816. [Google Scholar]
- Jedynak, K.; Wideł, D.; Rędzia, N. Removal of rhodamine b (a basic dye) and acid yellow 17 (an acidic dye) from aqueous solutions by ordered mesoporous carbon and commercial activated carbon. Colloids Interfaces 2019, 3, 30. [Google Scholar] [CrossRef]
- Al-Malack, M.H.; Basaleh, A.A. Adsorption of heavy metals using activated carbon produced from municipal organic solid waste organic solid waste. Desalin. Water Treat. 2016, 57, 24519–24531. [Google Scholar] [CrossRef]
- Fahmi, A.H.; Samsuri, A.W.; Jol, H.; Singh, D. Physical modification of biochar to expose the inner pores and their functional groups to enhance lead adsorption. RSC Adv. 2018, 8, 38270–38280. [Google Scholar] [CrossRef] [Green Version]
- Shu, Y.; Tang, C.; Hu, X.; Jiang, L.; Hu, X.; Zhao, Y. H3PO4-activated cattail carbon production and application in chromium removal from aqueous solution: Process optimization and removal mechanism. Water 2018, 10, 754. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Thapa, M.; Shrestha, R.G.; Maji, S.; Pradhananga, R.R.; Ariga, K. Rice husk-derived high surface area nanoporous carbon materials with excellent iodine and methylene blue adsorption properties. C 2019, 5, 10. [Google Scholar] [CrossRef]
- Yorgun, S.; Yıldız, D.; Şimşek, Y.E. Activated carbon from paulownia wood: Yields of chemical activation stages. Energy Sources A 2016, 38, 2035–2042. [Google Scholar] [CrossRef]
- Abdeljaoued, A.; Querejeta, N.; Durán, I.; Álvarez-Gutiérrez, N.; Pevida, C.; Chahbani, M. Preparation and evaluation of a coconut shell-Based activated carbon for CO2/CH4 separation. Energies 2018, 11, 1748. [Google Scholar] [CrossRef]
- Rahman, M.M.; Awang, M.; Shajahan, M.B.; Yunus, K.; Miskon, F.; Karim, M.R. Preparation of activated carbon by chemical activation and its in vitro adsorption efficacy tests for paraquat. Wulfenia J. 2014, 21, 237–242. [Google Scholar]
- Ioannidou, O.; Zabaniotou, A. Agricultural residues as precursors for activated carbon production—A review. Renew. Sustain. Energy Rev. 2007, 11, 1966–2005. [Google Scholar] [CrossRef]
- Pam, A.A.; Abdullah, A.H.; Tan, Y.P.; Zainal, Z. Batch and fixed bed adsorption of Pb (II) from aqueous solution using EDTA modified activated carbon derived from palm kernel shell. BioResources 2018, 13, 1235–1250. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Demiral, H.; Demiral, I.; Tümsek, F.; Karabacakoğlu, B. Pore structure of activated carbon prepared from hazelnut bagasse by chemical activation. Surf. Interface Anal. 2008, 40, 616–619. [Google Scholar] [CrossRef]
- Qiu, K.Q.; Yang, S.W.; Yang, J. Characteristics of activated carbon prepared from Chinese fir sawdust by zinc chloride activation under vacuum condition. J. Cent. South Univ. Technol. 2009, 16, 385–391. [Google Scholar] [CrossRef]
- Das, D.; Samal, D.P.; Meikap, B.C. Preparation of activated carbon from green coconut shell and its characterization. J. Chem. Eng. Process Technol. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, S.; Chong, K.H. Surface modification of a granular activated carbon by citric acid for enhancement of copper adsorption. Carbon 2003, 41, 1979–1986. [Google Scholar] [CrossRef]
- Kalpakli, Y.K.; Koyuncu, İ. Characterization of activated carbon and application of copper removal from drinking water. Ann. Chim. J. Anal. Environ. Cult. Herit. Chem. 2007, 97, 11–12. [Google Scholar] [CrossRef]
- Guijarro-Aldaco, A.; Hernandez-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A.; Mendoza-Castillo, D.I. Improving the adsorption of heavy metals from water using commercial carbons modified with egg shell wastes. Ind. Eng. Chem. Res. 2011, 50, 9354–9362. [Google Scholar] [CrossRef]
- Hanum, F.; Bani, O.; Wirani, L.I. Characterization of activated carbon from rice husk by HCl activation and its application for lead(Pb) removal in car battery wastewater. In IOP Conference Series: Materials Science and Engineering, Proceedings of 1st Annual Applied Science and Engineering Conference, Bandung, Indonesia, 18 November 2016; IOP Publishing: Bristol, UK, 2017; Volume 180, p. 012151. [Google Scholar]
- Huang, Y.; Li, S.; Lin, H.; Chen, J. Fabrication and characterization of mesoporous activated carbon from Lemna minor using one-step H3PO4 activation for Pb(II) removal. Appl. Surf. Sci. 2014, 317, 422–431. [Google Scholar] [CrossRef]




| Sample | Yield (%) | SBET (m2/g) | Micropore Volume (Vmic, cm3/g) | Total Pore Volume (cm3/g) | Average Pore Width (Å) |
|---|---|---|---|---|---|
| AC-600 2:3 | 30.1 | 1413 | 0.5980 | 0.6181 | 11.002 |
| AC-600 1:4 | 30.7 | 154.0 | 0.2850 | 0.3517 | 5.4697 |
| AC-500 2:3 | 55.5 | 337.2 | 0.3726 | 0. 3921 | 5.5264 |
| Precursor | Activating Agent | Temperature (°C) | SBET (m2/g) | Total Pore Volume (cm3/g) | References |
|---|---|---|---|---|---|
| Palm kernel shell | DES/H3PO4 | 600 | 1413 | 0.618 | This work |
| Rice husk | NaOH | 900 | 885 | 1.180 | [26] |
| Palm kernel shell | H3PO4 | 550–650 | 968–1153 | 0.677–0.711 | [33] |
| Hazelnut bagasse | KOH | 700 | 1642 | 0.964 | [34] |
| Hazelnut bagasse | ZnCl2 | 600 | 1489 | 0.933 | [34] |
| Rice husk | 700 | 750 | 0.380 | [21] | |
| Bagasse | 700 | 674 | 0.340 | [21] | |
| Chinese fir sawdust | ZnCl2 | 400–600 | 1071 | 0.566 | [35] |
| Green Coconut Shells | ZnCl2 | 650 | 996 | 0.448 | [36] |
| CA | 648 | - | [37] | ||
| CA | 825 | 0.696 | [38] | ||
| coconut shell carbon, CA | 399 | 0.172 | [29] | ||
| bituminous carbon, CA | 961 | 0.369 | [29] | ||
| lignite carbon, CA | 658 | 0.250 | [29] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pam, A.A. Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4. C 2019, 5, 43. https://doi.org/10.3390/c5030043
Pam AA. Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4. C. 2019; 5(3):43. https://doi.org/10.3390/c5030043
Chicago/Turabian StylePam, Aloysius Akaangee. 2019. "Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4" C 5, no. 3: 43. https://doi.org/10.3390/c5030043
APA StylePam, A. A. (2019). Innovative Activated Carbon Based on Deep Eutectic Solvents (DES) and H3PO4. C, 5(3), 43. https://doi.org/10.3390/c5030043