Synthesis of Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition Using Fe1−xMnxO Nanoparticles as Catalysts: How Does the Catalytic Activity of Graphitization Affect the Yields and Morphology?
Abstract
:1. Introduction
2. Materials and Methods
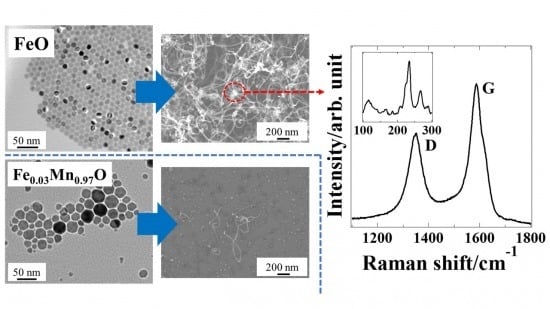
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603–605. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Hone, J.; Llaguno, M.; Biercuk, M.; Johnson, A.; Batlogg, B.; Benes, Z.; Fischer, J. Thermal properties of carbon nanotubes and nanotube-based materials. Appl. Phys. A 2002, 74, 339–343. [Google Scholar] [CrossRef]
- Wong, E.; Sheehan, P.; Lieber, C. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Franklin, A. Electronics: The road to carbon nanotube transistors. Nature 2013, 498, 443–444. [Google Scholar] [CrossRef] [PubMed]
- Tans, S.; Verschueren, A.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar] [CrossRef]
- Martel, R.; Schmidt, T.; Shea, H.; Hertel, T.; Avouris, P. Single-and multi-wall carbon nanotube field-effect transistors. Appl. Phys. Lett. 1998, 73, 2447–2449. [Google Scholar] [CrossRef]
- Zhong, G.; Iwasaki, T.; Honda, K.; Furukawa, Y.; Ohdomari, I.; Kawarada, H. Low Temperature Synthesis of Extremely Dense and Vertically Aligned Single-Walled Carbon Nanotubes. Jpn. J. Appl. Phys. 2005, 44, 1558–1561. [Google Scholar] [CrossRef]
- Nakai, Y.; Honda, K.; Yanagi, K.; Kataura, H.; Kato, T.; Yamamoto, T.; Maniwa, Y. Giant Seebeck coefficient in semiconducting single-wall carbon nanotube film. Appl. Phys. Express 2014, 7, 025103. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, T.; Ajayan, P. Large-scale synthesis of carbon nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Ando, Y.; Iijima, S. Preparation of Carbon Nanotubes by Arc-Discharge Evaporation. Jpn. J. Appl. Phys. 1993, 32, L107–L109. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.; Kim, S.; Rinzler, A.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.; Rinzler, A.; Nikolaev, P.; Thess, A.; Colbert, D.; Smalley, R. Single-wall nanotubes produced by metal-catalyzed disproportionation of carbon monoxide. Chem. Phys. Lett. 1996, 260, 471–475. [Google Scholar] [CrossRef]
- Bachilo, S.M.; Balzano, L.; Herrera, J.; Pompeo, F.; Resasco, D.; Weisman, B. Narrow (n, m)-Distribution of Single-Walled Carbon Nanotubes Grown Using a Solid Supported Catalyst. J. Am. Chem. Soc. 2003, 125, 11186–11187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tu, X.; Zaric, S.; Welsher, K.; Seo, W.; Zhao, W.; Dai, H. Selective synthesis combined with chemical separation of single-walled carbon nanotubes for chirality selection. J. Am. Chem. Soc. 2007, 129, 15770–15771. [Google Scholar] [CrossRef] [PubMed]
- Takagi, D.; Homma, Y.; Hibino, H.; Suzuki, S.; Kobayashi, Y. Single-walled carbon nanotube growth from highly activated metal nanoparticles. Nano Lett. 2006, 6, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Zhou, W.; Chu, H.; Jin, Z.; Zhang, Y.; Li, Y. Direct preparation and patterning of iron oxide nanoparticles via microcontact printing on silicon wafers for the growth of single-walled carbon nanotubes. Chem. Mater. 2006, 18, 4109–4114. [Google Scholar] [CrossRef]
- Yuan, D.; Ding, L.; Chu, H.; Feng, Y.; McNicholas, T.P.; Liu, J. Horizontally aligned single-walled carbon nanotube on quartz from a large variety of metal catalysts. Nano Lett. 2008, 8, 2576–2579. [Google Scholar] [CrossRef]
- Liu, B.; Ren, W.; Gao, L.; Li, S.; Liu, Q.; Jiang, C.; Cheng, H.M. Manganese-catalyzed surface growth of single-walled carbon nanotubes with high efficiency. J. Phys. Chem. C 2008, 112, 19231–19235. [Google Scholar] [CrossRef]
- Takagi, D.; Kobayashi, Y.; Hibino, H.; Suzuki, S.; Homma, Y. Mechanism of gold-catalyzed carbon material growth. Nano Lett. 2008, 8, 832–835. [Google Scholar] [CrossRef]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.H.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Norskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, P.; Bronikowski, M.; Bradley, R.; Rohmund, F.; Colbert, D.; Smith, K.; Smalley, R. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 1999, 313, 91–97. [Google Scholar] [CrossRef]
- Hata, H.; Futaba, D.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitiyanan, B.; Alvarez, W.; Harwell, J.; Resasco, D. Controlled production of single-wall carbon nanotubes by catalytic decomposition of CO on bimetallic Co–Mo catalysts. Chem. Phys. Lett. 2000, 317, 497–503. [Google Scholar] [CrossRef]
- Sanchez-Valencia, J.; Dienel, T.; Groning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.; Ruffieux, P.; Fasel, R. Controlled synthesis of single-chirality carbon nanotubes. Nature 2014, 512, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Wang, X.; Zhang, D.; Yang, J.; Luo, D.; Xu, Z.; Wei, J.; Wang, J.; Xu, Z.; Peng, F.; et al. Chirality-specific growth of single-walled carbon nanotubes on solid alloy catalysts. Nature 2014, 510, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Sharma, R.; Ducati, C.; Du, G.; Mattevi, C.; Cepek, C.; Cantoro, M.; Pisana, S.; Parvez, A.; Cervantes-Sodi, F.; et al. In situ Observations of Catalyst Dynamics during Surface-Bound Carbon Nanotube Nucleation. Nano Lett. 2007, 7, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Hongo, H.; Yudasaka, M.; Ichihashi, T.; Nihey, F.; Iijima, S. Chemical vapor deposition of single-wall carbon nanotubes on iron-film-coated sapphire substrates. Chem. Phys. Lett. 2002, 361, 349–354. [Google Scholar] [CrossRef]
- Kong, J.; Soh, H.; Cassell, A.; Quate, C.; Dai, H. Synthesis of individual single-walled carbon nanotubes on patterned silicon wafers. Nature 1998, 395, 878–881. [Google Scholar] [CrossRef]
- Su, M.; Zheng, B.; Liu, J. A scalable CVD method for the synthesis of single-walled carbon nanotubes with high catalyst productivity. Chem. Phys. Lett. 2000, 322, 321–326. [Google Scholar] [CrossRef]
- Ghorannevis, Z.; Kato, T.; Kaneko, T.; Hatakeyama, R. Growth of single-walled carbon nanotubes from nonmagnetic catalysts by plasma chemical vapor deposition. Jpn. J. Appl. Phys. 2010, 49, 02BA01. [Google Scholar] [CrossRef]
- Kato, T.; Hatakeyama, R. Direct growth of short single-walled carbon nanotubes with narrow-chirality distribution by time-programmed plasma chemical vapor deposition. ACS Nano 2010, 4, 7395–7400. [Google Scholar] [CrossRef] [PubMed]
- Meyyappan, M. A review of plasma enhanced chemical vapor deposition of carbon nanotubes. J. Phys. D 2009, 42, 213001. [Google Scholar] [CrossRef]
- Abdi, Y.; Mohajerzadeh, S.; Koohshorkhi, J.; Robertson, M.D.; Andrei, C.M. A plasma enhanced chemical vapor deposition process to achieve branched carbon nanotubes. Carbon 2008, 46, 1611–1614. [Google Scholar] [CrossRef]
- Hofmann, S.; Kleinsorge, B.; Ducati, C.; Ferrari, A.C.; Robertson, J. Low-temperature plasma enhanced chemical vapour deposition of carbon nanotubes. Diam. Relat. Mater. 2004, 13, 1171–1176. [Google Scholar] [CrossRef]
- Cole, M.T.; Milne, W.I. Plasma Enhanced Chemical Vapour Deposition of Horizontally Aligned Carbon Nanotubes. Materials 2013, 6, 2262–2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, Y.; Xu, Z.; Sun, S. Controlled Synthesis and Chemical Conversions of FeO Nanoparticles. Angewandte Chemie Int. Ed. 2007, 119, 6445–6448. [Google Scholar] [CrossRef]
- Donegá, C.M.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical evaluation of the hot-injection method, a synthesis route for monodisperse nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef]
- Peng, X.; Wickham, J.; Alivisatos, A. Kinetics of II-VI and III-V colloidal semiconductor nanocrystal growth: “Focusing” of size distributions. J. Am. Chem. Soc. 1998, 120, 5343–5344. [Google Scholar] [CrossRef]
- Shimada, T.; Miura, T.; Xie, W.; Yanase, T.; Nagahama, T. A thermocouple-based remote temperature controller of an electrically-floated sample for plasma CVD of nanocarbons with bias voltage. Measurement 2017, 102, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Kern, W. The Evolution of Silicon Wafer Cleaning Technology. J. Electrochem. Soc. 1990, 137, 1887–1892. [Google Scholar] [CrossRef]
- Zhong, G.; Iwasaki, T.; Robertson, J.; Kawarada, H. Growth Kinetics of 0.5 cm Vertically Aligned Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2007, 111, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Esconjauregui, S.; Fouquet, M.; Bayer, B.; Eslava, S.; Khachadorian, S.; Hofmann, S.; Robertson, J. Manipulation of the catalyst-support interactions for inducing nanotube forest growth. J. Appl. Phys. 2011, 109, 044303. [Google Scholar] [CrossRef]
- Shukrullah, S.; Mohamed, N.; Shaharun, M. Optimum temperature on structural growth of multiwalled carbon nanotubes with low activation energy. Diam. Relat. Mater. 2015, 58, 129–138. [Google Scholar] [CrossRef]
- Hernadi, K. Catalytic synthesis of multiwall carbon nanotubes from methylacetylene. Chem. Phys. Lett. 2002, 363, 169–174. [Google Scholar] [CrossRef]
- Panic, S.; Bajac, B.; Rakić, S.; Kónya, K.A.Z.; Boskovic, V.G. Molybdenum anchoring effect in Fe-Mo/MgO catalyst for multiwalled carbon nanotube synthesis. React. Kinet. Mech. Catal. 2017, 122, 775–791. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, K.; Jeong, S.; Park, M.; Yang, C.; Lee, Y. High-yield catalytic synthesis of thin multiwalled carbon nanotubes. J. Phys. Chem. B 2004, 108, 17695–17698. [Google Scholar] [CrossRef]
- Mizutani, K.; Kohno, H. Multi-walled carbon nanotubes with rectangular or square cross-section. Appl. Phys. Lett. 2016, 108, 263112. [Google Scholar] [CrossRef]
- DiLeo, R.; Landi, B.; Raffaelle, R. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Delhaes, P.; Couzi, M.; Trinquecoste, M.; Dentzer, J.; Hamidou, H.; Vix-Guterl, C. A comparison between Raman spectroscopy and surface characterizations of multiwall carbon nanotubes. Carbon 2006, 44, 3005–3013. [Google Scholar] [CrossRef]









| Fe(acac)3/mmol | Mn(acac)3/mmol | x by EDS | x by XRD | |
|---|---|---|---|---|
| (i) | 2.0 | 0 | 0 | 0 |
| (ii) | 1.0 | 1.0 | 0.5 | 0.5 |
| (iii) | 0.17 | 1.8 | 0.97 | 0.9 |
| (iv) | 0 | 2.0 | 1 | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanase, T.; Miura, T.; Shiratori, T.; Weng, M.; Nagahama, T.; Shimada, T. Synthesis of Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition Using Fe1−xMnxO Nanoparticles as Catalysts: How Does the Catalytic Activity of Graphitization Affect the Yields and Morphology? C 2019, 5, 46. https://doi.org/10.3390/c5030046
Yanase T, Miura T, Shiratori T, Weng M, Nagahama T, Shimada T. Synthesis of Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition Using Fe1−xMnxO Nanoparticles as Catalysts: How Does the Catalytic Activity of Graphitization Affect the Yields and Morphology? C. 2019; 5(3):46. https://doi.org/10.3390/c5030046
Chicago/Turabian StyleYanase, Takashi, Takuya Miura, Tatsuya Shiratori, Mengting Weng, Taro Nagahama, and Toshihiro Shimada. 2019. "Synthesis of Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition Using Fe1−xMnxO Nanoparticles as Catalysts: How Does the Catalytic Activity of Graphitization Affect the Yields and Morphology?" C 5, no. 3: 46. https://doi.org/10.3390/c5030046
APA StyleYanase, T., Miura, T., Shiratori, T., Weng, M., Nagahama, T., & Shimada, T. (2019). Synthesis of Carbon Nanotubes by Plasma-Enhanced Chemical Vapor Deposition Using Fe1−xMnxO Nanoparticles as Catalysts: How Does the Catalytic Activity of Graphitization Affect the Yields and Morphology? C, 5(3), 46. https://doi.org/10.3390/c5030046