Integration Methods of Cyclodextrins on Gold and Carbon Electrodes for Electrochemical Sensors
Abstract
:1. Introduction
2. Chemical Modifications of CDs for Electrochemical Devices
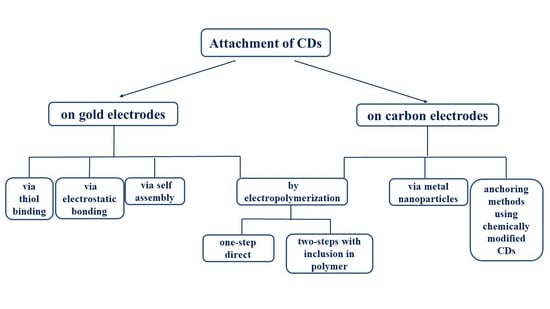
3. Immobilization Strategies on Electrode Surfaces
3.1. Inclusion of CDs onto Gold Electrode
3.1.1. Covalent Bond with SH-β-CDs
3.1.2. Electrostatic Bonding
3.1.3. Self-Assembly
3.2. Deposition Method for Both Gold and Carbon Electrode: Electropolymerization
3.2.1. Direct Electropolymerization of CDs
3.2.2. Inclusion of CDs into a Polymer Film
3.3. Inclusion of CDs onto Carbon Electrode
3.3.1. Load of Metal Nanoparticles on Carbon-Based/β-CDs Composites
3.3.2. Anchoring Methods Using Chemically Modified CDs
4. CDs for Biomedical Devices
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boero, C.; Casulli, M.A.; Olivo, J.; Foglia, L.; Orso, E.; Mazza, M.; Carrara, S.; De Micheli, G. Design, development, and validation of an in-situ biosensor array for metabolite monitoring of cell cultures. Biosens. Bioelectron. 2014, 61, 251–259. [Google Scholar] [CrossRef]
- Baj-Rossi, C.; De Micheli, G.; Carrara, S. Electrochemical detection of anti-breast-cancer agents in human serum by cytochrome P450-coated carbon nanotubes. Sensors 2012, 12, 6520–6537. [Google Scholar] [CrossRef]
- Park, S.; Boo, H.; Dong, T. Electrochemical non-enzymatic glucose sensors. Anal. Chim. Acta 2006, 556, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Baj-Rossi, C.; Rezzonico Jost, T.; Cavallini, A.; Grassi, F.; De Micheli, G.; Carrara, S. Continuous monitoring of Naproxen by a cytochrome P450-based electrochemical sensor. Biosens. Bioelectron. 2014, 53, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Mo, Z.; Yang, X.; Sun, M.; Zhao, P.; Li, Z.; Ouyang, M.; Liu, Z.; Gao, H.; Guo, R.; et al. Advances in the use of functional composites of β-cyclodextrin in electrochemical sensors. Microchim. Acta 2018, 185, 328. [Google Scholar] [CrossRef] [PubMed]
- Badruddoza, A.Z.M.; Si, G.; Hazel, S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 367, 85–95. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Tay, A.S.H.; Tan, P.Y.; Hidajat, K.; Uddin, M.S. Carboxymethyl-β-cyclodextrin conjugated magnetic nanoparticles as nano-adsorbents for removal of copper ions: Synthesis and adsorption studies. J. Hazard. Mater. 2011, 185, 1177–1186. [Google Scholar] [CrossRef]
- Wu, S.; Fan, S.; Tan, S.; Wang, J.; Li, C. A new strategy for the sensitive electrochemical determination of nitrophenol isomers using β-cyclodextrin derivative-functionalized silicon carbide. RSC Adv. 2018, 8, 775–784. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, J.; Tao, Y.; Wu, J.; Xie, C.; Kong, L. Three-dimensional mono-6-thio-β-cyclodextrin covalently functionalized gold nanoparticle/single-wall carbon nanotube hybrids for highly sensitive and selective electrochemical determination of methyl parathion. Electrochim. Acta 2015, 153, 12–18. [Google Scholar] [CrossRef]
- Rojas, M.T.; Konigety, R.; Stoddart, J.F.; Kaifer, A.E. Supported monolayers containing preformed binding sites. synthesis and interfacial binding properties of a thiolated/3-cyclodextrin derivative. J. Am. Chem. Soc. 1995, 117, 336–343. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Guo, Z.; Liu, J. Synthesis of novel decorated one-dimensional gold nanoparticle and its application in ultrasensitive detection of insecticide. J. Mater. Chem. 2010, 20, 5271–5279. [Google Scholar] [CrossRef]
- Weisser, M.; Nelles, G.; Wohlfart, P.; Wenz, G.; Mittler-Neher, S. Immobilization kinetics of cyclodextrins at gold surfaces. J. Phys. Chem. 1996, 3654, 17893–17900. [Google Scholar] [CrossRef]
- Maeda, Y.; Fukuda, T.; Yamamoto, H.; Kitano, H. Regio-and stereoselective complexation by a self-assembled monolayer of thiolated cyclodextrin on a gold electrode. Langmuir 1997, 13, 4187–4189. [Google Scholar] [CrossRef]
- Kitano, H.; Taira, Y.; Yamamoto, H. Inclusion of phthalate esters by a self-assembled monolayer of thiolated cyclodextrin on a gold electrode. Anal. Chem. 2000, 72, 2976–2980. [Google Scholar] [CrossRef]
- Maeda, Y.; Kitano, H. Inclusional complexation by cyclodextrins at the surface of silver as evidenced by surface-enhanced resonance Raman spectroscopy. J. Phys. Chem. 1995, 99, 487–488. [Google Scholar] [CrossRef]
- Li, X.; Zheng, L.; Wang, Y.; Zhang, N.; Lou, Y.; Xiao, T.; Liu, J. A novel electrocatalyst with high sensitivity in detecting glutathione reduced by 2-hydroxypropyl-β-cyclodextrin enveloped 10-methylphenothiazine. RSC Adv. 2015, 5, 71749–71755. [Google Scholar] [CrossRef]
- Ye, L.; Huang, N.; Du, Y.; Schneider, M.; Du, W. Succinyl-β-cyclodextrin modified gold biochip improved seroimmunological detection sensitivity for Lyme disease. Anal. Chim. Acta 2017, 953, 48–56. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, J.; Li, S.; Guo, M.; Fan, Z. An electrochemical sensor for the detection of: P -nitrophenol based on a cyclodextrin-decorated gold nanoparticle-mesoporous carbon hybrid. Analyst 2019, 144, 4400–4406. [Google Scholar] [CrossRef]
- Hea, P.; Fanga, Y.; Suzukib, I.; Osab, T. Voltammetric responsive sensors for organic compounds based on organized self-assembled lipoyl-P-cyclodextrin derivative monolayer on a gold electrode. Anal. Chim. Acta 1997, 337, 217–223. [Google Scholar] [CrossRef]
- Tang, B.; Liang, H.; Xu, K.; Mao, Z.; Shi, X.; Chen, Z. An improved synthesis of disulfides linked β-cyclodextrin dimer and its analytical application for dequalinium chloride determination by spectrofluorimetry. Anal. Chim. Acta 2005, 554, 31–36. [Google Scholar] [CrossRef]
- Liu, J.; Ong, W.; Román, E.; Lynn, M.J.; Kaifer, A.E. Cyclodextrin-modified gold nanospheres. Langmuir 2000, 16, 3000–3002. [Google Scholar] [CrossRef]
- Kong, L.; Wang, J.; Meng, F.; Chen, X.; Jin, Z.; Li, M.; Liu, J.; Huang, X.J. Novel hybridized SWCNT-PCD: Synthesis and host-guest inclusion for electrical sensing recognition of persistent organic pollutants. J. Mater. Chem. 2011, 21, 11109–11115. [Google Scholar] [CrossRef]
- Tan, L.; Wang, G.; Chen, N.; Zhang, J.; Feng, H. Layer-by-layer assembled multilayers of graphene/mono-(6-amino-6-deoxy)-β-cyclodextrin for detection of dopamine. Chin. J. Chem. 2015, 33, 185–191. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, Z.; Shi, M.; Yang, L.; Qiao, J.; Jiang, K. High-efficiency palladium nanoparticles supported on hydroxypropyl-β-cyclodextrin modified fullerene [60] for ethanol oxidation. Electrochim. Acta 2015, 177, 113–117. [Google Scholar] [CrossRef]
- Shang, F.; Zhou, L.; Mahmoud, K.A.; Hrapovic, S.; Liu, Y.; Moynihan, H.A.; Glennon, J.D.; Luong, J.H. Selective nanomolar detection of dopamine using a boron-doped diamond electrode modified with an electropolymerized sulfobutylether-β-cyclodextrin-doped poly (N-acetyltyramine) and polypyrrole composite film. Anal. Chem. 2009, 81, 4089–4098. [Google Scholar] [CrossRef]
- García, M.; Bollo, S.; Rivas, G.A.; Ferreyra, N.F.; Yáñez, C. Bottom up approaches for amino β-CD adsorption on gold surfaces. A comparative study. Electrochim. Acta 2016, 203, 292–300. [Google Scholar] [CrossRef]
- Di Palma, G.; Kotowska, A.M.; Hart, L.R.; Scurr, D.J.; Rawson, F.J.; Tommasone, S.; Mendes, P.M. Reversible, high-affinity surface capturing of proteins directed by supramolecular assembly. ACS Appl. Mater. Interfaces 2019, 11, 8937–8944. [Google Scholar] [CrossRef]
- Labuda, A.F.J. Cyclodextrins as electrode modifiers. Fresenius’ J. Anal. Chem. 2001, 370, 1–10. [Google Scholar]
- Godínez, L.A.; Lin, J.; Muñoz, M.; Coleman, A.W.; Rubin, S.; Parikh, A.; Zawodzinski, T.A.; Loveday, D.; Ferraris, J.P.; Kaifer, A.E. Multilayer self-assembly of amphiphilic cyclodextrin hosts on bare and modified gold substrates: Controlling aggregation via surface modification. Langmuir 1998, 14, 137–144. [Google Scholar]
- Veerbeek, J.; Méndez-Ardoy, A.; Huskens, J. Self-assembled monolayers of heptapodant β-cyclodextrins on gold. Langmuir 1998, 14, 6424–6429. [Google Scholar]
- Steentjes, T.; Kudernac, T.; Huskens, J. Self-assembled monolayers on gold of β-cyclodextrin adsorbates with different anchoring groups. Langmuir 2014, 30, 3467–3476. [Google Scholar]
- Méndez-Torres, A.M.; Sandoval-Altamirano, C.; Sánchez-Arenillas, M.; Marco, J.F.; Yáñez, C. Amino β-cyclodextrins immobilized on gold surfaces: Effect of substituents on host-guest interactions. Electrochim. Acta 2018, 282, 860–869. [Google Scholar]
- Yang, J.; Kim, H.T.; Kim, H. A cyclodextrin-based approach for selective detection of catecholamine hormone mixtures. Micro Nano Syst. Lett. 2014, 2, 1–10. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Liu, Y.; Zhang, X.; Chen, J. A sensitive electrochemical immunosensor for prion detection based on poly-β-cyclodextrin/gold nanoparticles/glassy carbon electrode. Sens. Actuators B Chem. 2017, 250, 1–7. [Google Scholar] [CrossRef]
- Abdorahim, M.; Rabiee, M.; Alhosseini, S.N.; Tahriri, M.; Yazdanpanah, S.; Alavi, S.H.; Tayebi, L. Nanomaterials-based electrochemical immunosensors for cardiac troponin recognition: An illustrated review. Trends Anal. Chem. 2016, 82, 337–347. [Google Scholar] [CrossRef]
- Hui, Y.; Ma, X.; Qu, F.; Chen, F.; Yu, J. Electropolymerization of carboxymethyl-β-cyclodextrin based on co-electrodeposition gold nanoparticles electrode: Electrocatalysis and nonenzymatic glucose sensing. J. Solid State Electrochem. 2016, 20, 1377–1389. [Google Scholar] [CrossRef]
- Souza, F.D.; Hsieh, Y.; Wickman, H.; Kutner, W. β-cyclodextrin and carboxymethylated β-cyclodextrin polymer film modified electrodes, hosting cobalt porphyrins, as sensors for electrocatalytic determination of oxygen dissolved in solution. Electroanalysis 1997, 9, 1093–1101. [Google Scholar] [CrossRef]
- Guo, Z.; Florea, A.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Zhang, A.; Săndulescu, R.; Lagarde, F.; Jaffrezic-Renault, N. 1, 3, 5-Trinitrotoluene detection by a molecularly imprinted polymer sensor based on electropolymerization of a microporous-metal-organic framework. Sens. Actuators B Chem. 2015, 207, 960–966. [Google Scholar] [CrossRef]
- Florea, A.; Guo, Z.; Cristea, C.; Bessueille, F.; Vocanson, F.; Goutaland, F.; Dzyadevych, S.; Săndulescu, R.; Jaffrezic-Renault, N. Anticancer drug detection using a highly sensitive molecularly imprinted electrochemical sensor based on an electropolymerized microporous metal organic framework. Talanta 2015, 138, 71–76. [Google Scholar] [CrossRef]
- Wu, T.; Wei, X.; Ma, X.; Li, J. Amperometric sensing of L-phenylalanine using a gold electrode modified with a metal organic framework, a molecularly imprinted polymer, and β-cyclodextrin-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 2901–2907. [Google Scholar] [CrossRef]
- Arjomandi, J.; Holze, R. Electrochemical preparation and in situ characterization of poly(3-methylpyrrole) and poly(3-methylpyrrole-cyclodextrin) films on gold electrodes. Open Chem. 2008, 6, 199–207. [Google Scholar] [CrossRef]
- Arjomandi, J.; Holze, R. In situ characterization of N-methylpyrrole and (N-methylpyrrole-cyclodextrin) polymers on gold electrodes in aqueous and nonaqueous solution. Synth. Met. 2007, 157, 1021–1028. [Google Scholar] [CrossRef]
- Bidan, G.; Lopez, C.; Vieil, E. Incorporation of suiphonated cyclodextrins into polypyrrole: An approach for the electro-controlled delivering of neutral drugs. Biosens. Bioelectron. 1994, 9, 219–229. [Google Scholar]
- Dermody, D.L.; Peez, R.F.; Bergbreiter, D.E.; Crooks, R.M. Chemically grafted polymeric filters for chemical sensors: Hyperbranched poly (acrylic acid) films incorporating -cyclodextrin receptors and amine-functionalized filter layers. Langmuir 1999, 15, 885–890. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, S.; Lin, X.; Nie, L.; Rui, L. Preparation and characterization of a novel -cyclodextrin modified poly (N -acetylaniline) film. Macromolecules 2002, 6174–6177. [Google Scholar] [CrossRef]
- Abbaspour, A.; Noori, A. A cyclodextrin host–guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosens. Bioelectron. 2011, 26, 4674–4680. [Google Scholar] [CrossRef]
- Tao, Y.; Dai, J.; Kong, Y.; Sha, Y. Temperature-sensitive electrochemical recognition of tryptophan enantiomers based on β-cyclodextrin self-assembled on poly(l-glutamic acid). Anal. Chem. 2014, 86, 2633–2639. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, G.; Zhang, M. Electrochemical sensor based on electro-polymerization of β-cyclodextrin and reduced-graphene oxide on glassy carbon electrode for determination of gatifloxacin. Sens. Actuators B Chem. 2016, 228, 59–65. [Google Scholar] [CrossRef]
- Zhang, F.; Gu, S.; Ding, Y.; Zhang, Z.; Li, L. A novel sensor based on electropolymerization of β-cyclodextrin and l-arginine on carbon paste electrode for determination of fluoroquinolones. Anal. Chim. Acta 2013, 770, 53–61. [Google Scholar] [CrossRef]
- Bouchta, D.; Izaoumen, N.; Zejli, H.; El Kaoutit, M.; Temsamani, K.R. A novel electrochemical synthesis of poly-3-methylthiophene-gamma-cyclodextrin film. Application for the analysis of chlorpromazine and some neurotransmitters. Biosens. Bioelectron. 2005, 20, 2228–2235. [Google Scholar] [CrossRef]
- Sun, Y.; Wei, T.; Jiang, M.; Xu, L.; Xu, Z. Voltammetric sensor for chloramphenicol determination based on a dual signal enhancement strategy with ordered mesoporous carbon@polydopamine and β-cyclodextrin. Sens. Actuators B Chem. 2018, 255, 2155–2162. [Google Scholar] [CrossRef]
- Zhu, G.; Yi, Y.; Chen, J. Recent advances for cyclodextrin-based materials in electrochemical sensing. Trends Anal. Chem. 2016, 80, 232–241. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S. A novel amperometric nitrite sensor based on screen printed carbon electrode modified with graphite/β-cyclodextrin composite. J. Electroanal. Chem. 2016, 760, 97–104. [Google Scholar] [CrossRef]
- Zaidi, S.A. Facile and efficient electrochemical enantiomer recognition of phenylalanine using β-cyclodextrin immobilized on reduced graphene oxide. Biosens. Bioelectron. 2017, 94, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Kushikawa, R.T.; Silva, M.R.; Angelo, A.C.D.; Teixeira, M.F.S. Construction of an electrochemical sensing platform based on platinum nanoparticles supported on carbon for tetracycline determination. Sens. Actuators B Chem. 2016, 228, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Palanisamy, S.; Kokulnathan, T.; Chen, S.; Velusamy, V.; Kannan, S. Voltammetric determination of Sudan I in food samples based on platinum nanoparticles decorated on graphene-β-cyclodextrin modified electrode. J. Electroanal. Chem. 2017, 794, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Cheng, C.; Yuan, H.; Du, J.; Xiao, D.; Xie, S.; Choi, M.M. Simultaneous determination of L-ascorbic acid, dopamine and uric acid with gold nanoparticles-β-cyclodextrin-graphene-modified electrode by square wave voltammetry. Talanta 2012, 93, 79–85. [Google Scholar] [CrossRef]
- Zhu, G.; Gai, P.; Yang, Y.; Zhang, X.; Chen, J. Electrochemical sensor for naphthols based on gold nanoparticles/hollow nitrogen-doped carbon microsphere hybrids functionalized with. Anal. Chim. Acta 2012, 723, 33–38. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Gan, N.; Hu, F. An on-site immunosensor for ractopamine based on a personal glucose meter and using magnetic β-cyclodextrin-coated nanoparticles for enrichment, and an invertase-labeled nanogold probe for signal amplification. Microchim. Acta 2015, 182, 815–822. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, X.; Gai, P.; Zhang, X.; Chen, J. β-Cyclodextrin non-covalently functionalized single-walled carbon nanotubes bridged by 3, 4, 9, 10-perylene tetracarboxylic acid for ultrasensitive electrochemical sensing of 9-anthracenecarboxylic acid. Nanoscale 2012, 4, 5703–5709. [Google Scholar] [CrossRef]
- Chem, J.M.; Xu, C.; Wang, J.; Wan, L.; Lin, J.; Wang, X. Microwave-assisted covalent modification of graphenenanosheets with hydroxypropyl-β-cyclodextrin and its electrochemical detection of phenolic organic pollutants. J. Mater. Chem. 2011, 21, 10463–10471. [Google Scholar]
- Zhu, G.; Yi, Y.; Liu, Z.; Jin, H.; Chen, J. Electrochemistry communications highly sensitive electrochemical sensing based on 2-hydroxypropyl-β-cyclodextrin-functionalized graphene nanoribbons. Electrochem. Commun. 2016, 66, 10–15. [Google Scholar] [CrossRef]
- Yang, L.; Fan, S.; Deng, G.; Li, Y.; Ran, X.; Zhao, H. Bridged β-cyclodextrin-functionalized MWCNT with higher supramo- lecular recognition capability: The simultaneous electrochemical determination of three phenols. Biosens. Bioelectron. 2015, 68, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Y.; Hu, L.; Stoddart, J.F.; Gru, G. Pyrenecyclodextrin-decorated single-walled carbon nanotube field-effect transistors as chemical sensors. Adv. Mater. 2008, 20, 1910–1915. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, L.; Yang, R.; Wang, L.; Liu, J.; Huang, X. Single-walled carbon nanotube/pyrenecyclodextrin nanohybrids for ultrahighly sensitive and selective detection of p -nitrophenol. Langmuir 2011, 27, 10295–10301. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, H.; Li, C.; Fan, S.; Li, B. Dual β-cyclodextrin functionalized Au@SiC nanohybrids for the electrochemical determination of tadalafil in the presence of acetonitrile. Biosens. Bioelectron. 2015, 64, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Cova, T.F.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M. Combining cellulose and cyclodextrins: Fascinating designs for materials and pharmaceutics. Front. Chem. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Gao, J.; Guo, Z.; Su, F.; Gao, L.; Pang, X.; Cao, W.; Du, B.; Wei, Q. Ultrasensitive electrochemical immunoassay for CEA through host–guest interaction of β-cyclodextrin functionalized graphene and Cu@Ag core–shell nanoparticles with adamantine-modified antibody. Biosens. Bioelectron. 2015, 63, 465–471. [Google Scholar] [CrossRef]
- Koradecki, D.; Kutner, W. Inclusion of the regioisomeric nitrobenzene derivatives and ferrocene guests by β-cyclodextrin polymer and their transport through the polymer matrix. J. Incl. Phenom. Mol. Recognit. Chem. 1991, 79–96. [Google Scholar] [CrossRef]
- Ju, H. Host-guest interaction at a self-assembled monolayer/solution interface: An electrochemical analysis of the inclusion of 11-(Ferrocenylcarbonyloxy)undecanethiol by cyclodextrins. Langmuir 1998, 14, 300–306. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, L.; Zhang, X.; Liu, W.; Zhang, X.; Chen, J. A New dual-signalling electrochemical sensing strategy based on competitive host-guest interaction of a b -cyclodextrin/poly (N-acetylaniline)/graphene-modified electrode: Sensitive electrochemical determination of organic pollutants. Chem. Eur. J. 2013, 19, 6368–6373. [Google Scholar] [CrossRef] [PubMed]
- Hishiya, T.; Shibata, M.; Kakazu, M.; Asanuma, H.; Komiyama, M. Molecularly imprinted cyclodextrins as selective receptors for steroids. Macromolecules 1999, 32, 2265–2269. [Google Scholar] [CrossRef]









| CD Derivative | Kind of Interaction | CDs Integration Substrate |
|---|---|---|
| CM-CDs | electrostatic interaction amide linkage | gold |
| NH-CDs | electrostatic interaction amide linkage | gold/carbon |
| SH-CDs | Au-SH linkage | gold |
| HP-CDs | electrostatic interaction | carbon |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casulli, M.A.; Taurino, I.; Carrara, S.; Hayashita, T. Integration Methods of Cyclodextrins on Gold and Carbon Electrodes for Electrochemical Sensors. C 2019, 5, 78. https://doi.org/10.3390/c5040078
Casulli MA, Taurino I, Carrara S, Hayashita T. Integration Methods of Cyclodextrins on Gold and Carbon Electrodes for Electrochemical Sensors. C. 2019; 5(4):78. https://doi.org/10.3390/c5040078
Chicago/Turabian StyleCasulli, Maria Antonietta, Irene Taurino, Sandro Carrara, and Takashi Hayashita. 2019. "Integration Methods of Cyclodextrins on Gold and Carbon Electrodes for Electrochemical Sensors" C 5, no. 4: 78. https://doi.org/10.3390/c5040078
APA StyleCasulli, M. A., Taurino, I., Carrara, S., & Hayashita, T. (2019). Integration Methods of Cyclodextrins on Gold and Carbon Electrodes for Electrochemical Sensors. C, 5(4), 78. https://doi.org/10.3390/c5040078