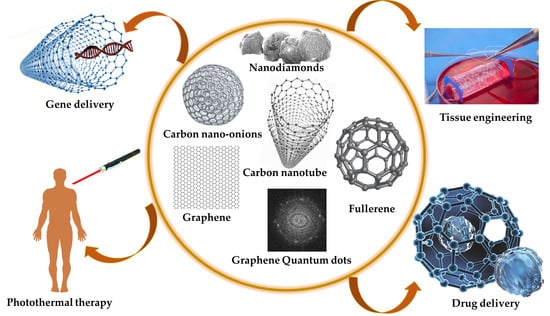
Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology
Abstract
:1. Introduction
- Their supramolecular π–π stacking attribute allows them to adsorb a high amount of drug.
- As a result of their unique optical characteristics and facile amalgamation with luminous substances, CNMs can be utilized as theranostics materials.
- CNMs possess excellent heat conversion capacity in the near-infrared region that could be well utilized for photothermal therapy.
- Tuneable surface chemistry can be used for the controlled release of therapeutics.
2. Carbon Nanotubes
2.1. Techniques for the Fabrication of Carbon Nanotubes
2.2. Functionalization of Carbon Nanotubes
- I.
- Covalent functionalization: Covalent modifications can be achieved by the direct sidewall functionalization or by defect group functionalization, which involves converting or rehybridization of sp2 carbon into sp3 configurations and finally forming covalency with attacking species [54]. This is either accomplished by using halogenation, the cycloaddition of azomethine ylides, and by the addition of radicals. The defect group surface modifications are finished by engendering defects by oxidation to yield carboxylic acid functionalities, which render them the functionality to attach various targeting groups such as peptides, proteins, or antibodies by employing amidation or esterification. Double and triple covalent functionalization is used for the fabrication of multifunctional CNTs that can be utilized for drug delivery. The double covalent functionalization can be achieved either by 1,3-dipolar cycloaddition and esterification/amidation and cyclopropanation; double arylation; arylation and amidation; amidation and cyclopropanation; double 1,3-dipolar cycloaddition; 1,3-dipolar cycloaddition and arylation; etc. The triple functionalization can be accomplished by simultaneous functionalization with different aryl diazonium salts [55,56].
- II.
- Non-covalent functionalization: The covalent functionalization achieved by the aforesaid modifications may lead to the loss of electrical and optical characteristics of carbon nanomaterials. Therefore, non-covalent functionalization methods were used that can avert the negative effects of the covalent modifications of CNTs. Non-covalent functionalization can be done by adsorption/wrapping biopolymers, surfactants, and polymers on the tubular surface via π–π stacking and van der Waals interactions. Polymers—for example, polyethylene glycol (PEG), poly(vinylpyrrolidone), tetraalkylammonium, poly (meta phenylene vinylene), etc.—are utilized to form a case around the single-walled carbon nanotubes when suspended in a solution of the polymer. Surfactants such as sodium dodecyl sulfate, sodium dodecylbenzene sulfonate (SDBS), Triton X-100, etc., aid in the improvement of dispersibility, solubility, and permeability through gastrointestinal tract (GIT) by adsorbing on the surface of CNTs via π interactions [57,58].
2.3. CNTs for Drug Delivery
2.4. CNTs for Vaccine Delivery
2.5. CNTs for Gene Delivery
3. Graphene/Graphene Oxide/Reduced Graphene Oxide
3.1. Techniques for the Fabrication of Graphene
3.2. Functionalization of Graphene
3.3. Graphene Oxide for Drug Delivery
3.4. Graphene Oxide for Gene Delivery
4. Graphene Quantum Dots (GQDs)
GQDs for Drug Delivery
5. Fullerenes
5.1. Techniques for the Fabrication of Fullerenes
5.2. Functionalization of Fullerenes
5.3. Fullerenes for Drug Delivery
5.4. Fullerenes for Antibody/Antiviral Delivery
6. Nanodiamonds
Nanodiamonds for the Delivery of Therapeutics
7. Carbon Nano-Onions
Carbon Nano-Onions for Therapeutics Delivery
8. Toxicity Concerns for Carbon-Based Nanomaterials
9. Conclusions
10. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging carriers for drug delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partha, R.; Conyers, J.L. Biomedical applications of functionalized fullerene-based nanomaterials. Int. J. Nanomed. 2009, 4, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Perkins, B.L.; Naderi, N. Carbon Nanostructures in Bone Tissue Engineering. Open Orthop. J. 2016, 10, 877–899. [Google Scholar] [CrossRef] [Green Version]
- Erol, O.; Uyan, I.; Hatip, M.; Yilmaz, C.; Tekinay, A.B.; Guler, M.O. Recent advances in bioactive 1D and 2D carbon nanomaterials for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2018, 4, 2433–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasnain, M.S.; Nayak, A.K. Background: Carbon nanotubes for targeted drug delivery. In SpringerBriefs in Applied Sciences and Technology; Springer: Singapore, 2019; pp. 1–9. ISBN 978-981-15-0910-0. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing graphene quantum dots and carbon dots: Properties, syntheses, and biological applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugarte, D. Onion-like graphitic particles. Carbon N. Y. 1995, 33, 989–993. [Google Scholar] [CrossRef]
- Generalic, E. “Allotrope.” Croatian-English Chemistry Dictionary & Glossary. Available online: https://glossary.periodni.com (accessed on 30 December 2020).
- Welcome To Cheap Tubes. Available online: https://www.cheaptubes.com (accessed on 30 December 2020).
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, S.I.; Song, G.Y.; Kim, I.I. Non-covalently functionalized carbon nanostructures for synthesizing carbon-based hybrid nanomaterials. J. Nanosci. Nanotechnol. 2014, 4, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Xu, Y.H.; Li, K.; Wang, C.; Liu, X.; Yue, Y.; Chen, Z.; Yuan, S.J.; Wen, Y.; Zhang, Q.; et al. Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019, 86, 381–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C 2017, 77, 1363–1377. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef]
- Yang, D.; Feng, L.; Dougherty, C.A.; Luker, K.E.; Chen, D.; Cauble, M.A.; Banaszak Holl, M.M.; Luker, G.D.; Ross, B.D.; Liu, Z.; et al. In vivo targeting of metastatic breast cancer via tumor vasculature-specific nano-graphene oxide. Biomaterials 2016, 104, 361–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Wu, R.; Zhao, L.; Wu, M.; Yang, L.; Zou, H. P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells. ACS Nano 2010, 4, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.W.; Kyung Lee, Y.; Yeon Lee, J.; Hee Hong, J.; Khang, D. PEGylated anticancer-carbon nanotubes complex targeting mitochondria of lung cancer cells. Nanotechnology 2017, 28, 465102. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, Y.; Zhao, M. Target-directed functionalized ferrous phosphate-carbon dots fluorescent nanostructures as peroxidase mimetics for cancer cell detection and ROS-mediated therapy. Sens. Actuators B Chem. 2019, 297, 126739. [Google Scholar] [CrossRef]
- Jiang, B.P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. A Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef]
- Wang, H.; Bi, J.; Zhu, B.-W.; Tan, M. Multicolorful Carbon Dots for Tumor Theranostics. Curr. Med. Chem. 2017, 25, 2894–2909. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Jović, D.; Jaćević, V.; Kuča, K.; Borišev, I.; Mrdjanovic, J.; Petrovic, D.; Seke, M.; Djordjevic, A. The puzzling potential of carbon nanomaterials: General properties, application, and toxicity. Nanomaterials 2020, 10, 1508. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef] [Green Version]
- Rehman, A.; Houshyar, S.; Wang, X. Nanodiamond in composite: Biomedical application. J. Biomed. Mater. Res. Part A 2020, 108, 906–922. [Google Scholar] [CrossRef]
- Ahlawat, J.; Masoudi Asil, S.; Guillama Barroso, G.; Nurunnabi, M.; Narayan, M. Application of carbon nano onions in the biomedical field: Recent advances and challenges. Biomater. Sci. 2021. [Google Scholar] [CrossRef]
- Kushwaha, S.K.S.; Ghoshal, S.; Rai, A.K.; Singh, S. Carbon nanotubes as a novel drug delivery system for anticancer therapy: A review. Braz. J. Pharm. Sci. 2013, 49, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M. BioMEMS and biomedical nanotechnology. In BioMEMS and Biomedical Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 2–17. ISBN 0387255664. [Google Scholar]
- Tsang, S.C.; Chen, Y.K.; Harris, P.J.F.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Ebbesen, T.W.; Ichihashi, T.; Iijima, S.; Tanigaki, K.; Hiura, H. Opening carbon nanotubes with oxygen and implications for filling. Nature 1993, 362, 522–525. [Google Scholar] [CrossRef]
- Ebbesen, T.W. Wetting, filling and decorating carbon nanotubes. J. Phys. Chem. Solids 1996, 57, 951–955. [Google Scholar] [CrossRef]
- Gao, H.; Kong, Y.; Cui, D.; Ozkan, C.S. Spontaneous insertion of DNA oligonucleotides into carbon nanotubes. Nano Lett. 2003, 3, 471–473. [Google Scholar] [CrossRef]
- Fu, Q.; Weinberg, G.; Su, D.S. Selective filling of carbon nanotubes with metals by selective washing. Xinxing Tan Cailiao New Carbon Mater. 2008, 23, 17–20. [Google Scholar] [CrossRef] [Green Version]
- De Jonge, N.; Doytcheva, M.; Allioux, M.; Kaiser, M.; Mentink, S.A.M.; Teo, K.B.K.; Lacerda, R.G.; Milne, W.I. Cap closing of thin carbon nanotubes. Adv. Mater. 2005, 17, 451–455. [Google Scholar] [CrossRef]
- Monthioux, M. Filling single-wall carbon nanotubes. Carbon N. Y. 2002, 40, 1809–1823. [Google Scholar] [CrossRef]
- Van den Broeck, L.; Piluso, S.; Soultan, A.H.; De Volder, M.; Patterson, J. Cytocompatible carbon nanotube reinforced polyethylene glycol composite hydrogels for tissue engineering. Mater. Sci. Eng. C 2019, 98, 1133–1144. [Google Scholar] [CrossRef]
- Zhang, P.; Yi, W.; Hou, J.; Yoo, S.; Jin, W.; Yang, Q. A carbon nanotube-gemcitabine-lentinan three-component composite for chemo-photothermal synergistic therapy of cancer. Int. J. Nanomed. 2018, 13, 3069–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komane, P.P.; Kumar, P.; Marimuthu, T.; du Toit, L.C.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. Dexamethasone-loaded, pegylated, vertically aligned, multiwalled carbon nanotubes for potential ischemic stroke intervention. Molecules 2018, 23, 1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, N.; Wang, M.; Jin, Y.; Ding, J.; Gao, X.; Sun, X.; Zhang, H.; Cui, M.; Zheng, J.; Li, N.; et al. Magnetic multiwalled carbon nanotubes with controlled release of epirubicin: An intravesical instillation system for bladder cancer. Int. J. Nanomed. 2019, 2019, 1241–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtari, J.; Faridnia, R.; Kalani, H.; Bastani, R.; Fakhar, M.; Rezvan, H.; Beydokhti, A.K. Potent in vitro antileishmanial activity of a nanoformulation of cisplatin with carbon nanotubes against Leishmania major. J. Glob. Antimicrob. Resist. 2019, 16, 11–16. [Google Scholar] [CrossRef]
- Chegeni, M.; Rozbahani, Z.S.; Ghasemian, M.; Mehri, M. Synthesis and application of the calcium alginate/SWCNT-Gl as a bio-nanocomposite for the curcumin delivery. Int. J. Biol. Macromol. 2020, 156, 504–513. [Google Scholar] [CrossRef]
- Maleki, R.; Afrouzi, H.H.; Hosseini, M.; Toghraie, D.; Rostami, S. Molecular dynamics simulation of Doxorubicin loading with N-isopropyl acrylamide carbon nanotube in a drug delivery system. Comput. Methods Programs Biomed. 2020, 184, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kavosi, A.; Hosseini Ghale Noei, S.; Madani, S.; Khalighfard, S.; Khodayari, S.; Khodayari, H.; Mirzaei, M.; Kalhori, M.R.; Yavarian, M.; Alizadeh, A.M.; et al. The toxicity and therapeutic effects of single-and multi-wall carbon nanotubes on mice breast cancer. Sci. Rep. 2018, 8, 8375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, S.Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.H.; Strano, M.S. Chloroplast-selective gene delivery and expression in planta using chitosan-complexed single-walled carbon nanotube carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef]
- Ohta, T.; Hashida, Y.; Yamashita, F.; Hashida, M. Development of Novel Drug and Gene Delivery Carriers Composed of Single-Walled Carbon Nanotubes and Designed Peptides With PEGylation. J. Pharm. Sci. 2016, 105, 2815–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Carbon nanotubes: A novel material for multifaceted applications in human healthcare. Chem. Soc. Rev. 2017, 158–196. [Google Scholar] [CrossRef] [PubMed]
- Herlem, G.; Picaud, F.; Girardet, C.; Micheau, O. Carbon Nanotubes: Synthesis, Characterization, and Applications in Drug-Delivery Systems. In Nanocarriers for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 469–529. ISBN 9780128140338. [Google Scholar]
- Kumar, N.; Kumbhat, S. Essentials in Nanoscience and Nanotechnology. In Essentials in Nanoscience and Nanotechnology; Wiley: Hoboken, NJ, USA, 2016; pp. 29–76. ISBN 9781119096122. [Google Scholar]
- Elhissi, A.M.A.; Ahmed, W.; Hassan, I.U.; Dhanak, V.R.; D’Emanuele, A. Carbon Nanotubes in Cancer Therapy and Drug Delivery. J. Drug Deliv. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Dai, H. Carbon nanotubes: Synthesis, integration, and properties. Acc. Chem. Res. 2002, 35, 1035–1044. [Google Scholar] [CrossRef]
- Dinesh, B.; Bianco, A.; Ménard-Moyon, C. Designing multimodal carbon nanotubes by covalent multi-functionalization. Nanoscale 2016, 8, 18596–18611. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kiran, A.V.V.V.; Kusuma Kumari, G.; Krishnamurthy, P.T. Carbon nanotubes in drug delivery: Focus on anticancer therapies. J. Drug Deliv. Sci. Technol. 2020, 59, 1–12. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Sinani, V.A.; Gheith, M.K.; Yaroslavov, A.A.; Rakhnyanskaya, A.A.; Sun, K.; Mamedov, A.A.; Wicksted, J.P.; Kotov, N.A. Aqueous dispersions of single-wall and multiwall carbon nanotubes with designed amphiphilic polycations. J. Am. Chem. Soc. 2005, 127, 3463–3472. [Google Scholar] [CrossRef]
- Iijima, S. Carbon nanotubes: Past, present, and future. Phys. B Condens. Matter 2002, 323, 1–5. [Google Scholar] [CrossRef]
- Hiraoka, T.; Bandow, S.; Shinohara, H.; Iijima, S. Control on the diameter of single-walled carbon nanotubes by changing the pressure in floating catalyst CVD. Carbon N. Y. 2006, 44, 1853–1859. [Google Scholar] [CrossRef]
- Xue, Y. Carbon Nanotubes for Biomedical Applications. In Industrial Applications of Carbon Nanotubes; William Andrew: Norwich, NY, USA, 2017; pp. 323–346. ISBN 9780323415316. [Google Scholar]
- Hwang, Y.; Park, S.H.; Lee, J.W. Applications of functionalized carbon nanotubes for the therapy and diagnosis of cancer. Polymers 2017, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanbhag, V.K.L.; Prasad, K.S. Graphene based sensors in the detection of glucose in saliva-a promising emerging modality to diagnose diabetes mellitus. Anal. Methods 2016, 8, 6255–6259. [Google Scholar] [CrossRef]
- Kim, P.; Odom, T.W.; Huang, J.L.; Lieber, C.M. Electronic density of states of atomically resolved single-walled carbon nanotubes: Van hove singularities and end states. Phys. Rev. Lett. 1999, 82, 1225–1228. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.; Lambin, P. Electronic structure of carbon nanotubes with chiral symmetry. Phys. Rev. B Condens. Matter Mater. Phys. 1998, 57, R15 037–R15 039. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the role of carbon nanotube composites in preventing biofouling and promoting biofilms on material surfaces in environmental engineering: A review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.S.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Guo, W.; Lin, Y.; Deng, X.Y.; Wang, H.F.; Sun, H.F.; Liu, Y.F.; Wang, X.; Wang, W.; Chen, M.; et al. Biodistribution of pristine single-walled carbon nanotubes in vivo. J. Phys. Chem. C 2007, 111, 17761–17764. [Google Scholar] [CrossRef]
- Saikia, N. Functionalized Carbon Nanomaterials in Drug Delivery: Emergent Perspectives from Application. In Novel Nanomaterials—Synthesis and Applications; IntechOpen: London, UK, 2018; pp. 231–255. ISBN 978-1-83881-460-1. [Google Scholar]
- Saikia, N.; Deka, R.C. A comparison of the effect of nanotube chirality and electronic properties on the π-π Interaction of single-wall carbon nanotubes with pyrazinamide antitubercular drug. Int. J. Quantum Chem. 2013, 113, 1272–1284. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, H.S.; Shin, U.S. Thermo conductive carbon nanotube-framed membranes for skin heat signal-responsive transdermal drug delivery. Polym. Chem. 2017, 8, 3154–3163. [Google Scholar] [CrossRef]
- Asghar, W.; Shafiee, H.; Velasco, V.; Sah, V.R.; Guo, S.; El Assal, R.; Inci, F.; Rajagopalan, A.; Jahangir, M.; Anchan, R.M.; et al. Toxicology Study of Single-walled Carbon Nanotubes and Reduced Graphene Oxide in Human Sperm. Sci. Rep. 2016, 6, 30270. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.J.; Gupta, B.K.; Chandra, P. Probing the electrical and dielectric properties of polyaniline multi-walled carbon nanotubes nanocomposites doped in different protonic acids. Polym. Bull. 2020, 1–17. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, C.; Yue, L.; Qiao, K.; Wang, G.; Wang, L.; Quan, H. Dual stimulus responsive drug release under the interaction of pH value and pulsatile electric field for a bacterial cellulose/sodium alginate/multi-walled carbon nanotube hybrid hydrogel. RSC Adv. 2015, 5, 41820–41829. [Google Scholar] [CrossRef]
- Seyfoori, A.; Sarfarazijami, S.; Seyyed Ebrahimi, S.A. pH-responsive carbon nanotube-based hybrid nanogels as the smart anticancer drug carrier. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Mehrjouei, E.; Akbarzadeh, H.; Shamkhali, A.N.; Abbaspour, M.; Salemi, S.; Abdi, P. Delivery of Cisplatin Anti-Cancer Drug from Carbon, Boron Nitride, and Silicon Carbide Nanotubes Forced by Ag-Nanowire: A Comprehensive Molecular Dynamics Study. Mol. Pharm. 2017, 14, 2273–2284. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Erfan, M.; Mortazavi, S.A.; Ghorbani-Bidkorbeh, F.; Kobarfard, F.; Shirazi, F.H. Functionalisation of carbon nanotubes by methotrexate and study of synchronous photothermal effect of carbon nanotube and anticancer drug on cancer cell death. IET Nanobiotechnol. 2019, 13, 52–57. [Google Scholar] [CrossRef]
- Sheikh, A.H.; Khalid, A.; Khan, F.; Begum, A. Fluorescent Gadolinium(III)-Oligopeptide Complexes and Carbon Nanotube Composite as Dual Modality Anticancer Agents. ChemistrySelect 2019, 4, 228–235. [Google Scholar] [CrossRef]
- Kabanov, A.V. Polymer genomics: An insight into pharmacology and toxicology of nanomedicines. Adv. Drug Deliv. Rev. 2006, 30, 1597–1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanone, S.; Boczkowski, J. Biomedical Applications and Potential Health Risks of Nanomaterials: Molecular Mechanisms. Curr. Mol. Med. 2006, 6, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzym. Regul. 2001, 41, 189–201. [Google Scholar] [CrossRef]
- Chu, Y.; Tang, D.; Ke, Z.; Ma, J.; Li, R. Polyethylenimine-functionalized multiwalled carbon nanotube for the adsorption of hydrogen sulfide. J. Appl. Polym. Sci. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Singh, N.; Sachdev, A.; Gopinath, P. Polysaccharide Functionalized Single Walled Carbon Nanotubes as Nanocarriers for Delivery of Curcumin in Lung Cancer Cells. J. Nanosci. Nanotechnol. 2017, 18, 1534–1541. [Google Scholar] [CrossRef]
- Jogi, H.; Maheshwari, R.; Raval, N.; Kuche, K.; Tambe, V.; Mak, K.K.; Pichika, M.R.; Tekade, R.K. Carbon nanotubes in the delivery of anticancer herbal drugs. Nanomedicine 2018, 13, 1187–1220. [Google Scholar] [CrossRef] [PubMed]
- Rathod, V.; Tripathi, R.; Joshi, P.; Jha, P.K.; Bahadur, P.; Tiwari, S. Paclitaxel Encapsulation into Dual-Functionalized Multi-Walled Carbon Nanotubes. AAPS PharmSciTech 2019, 20, 1–13. [Google Scholar] [CrossRef]
- Hossein Panahi, F.; Peighambardoust, S.J.; Davaran, S.; Salehi, R. Development and characterization of PLA-mPEG copolymer containing iron nanoparticle-coated carbon nanotubes for controlled delivery of Docetaxel. Polymer 2017, 117, 117–131. [Google Scholar] [CrossRef]
- Li, Z.; Tozer, T.; Alisaraie, L. Molecular Dynamics Studies for Optimization of Noncovalent Loading of Vinblastine on Single-Walled Carbon Nanotube. J. Phys. Chem. C 2016, 120, 4061–4070. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Bian, X.; Zhu, Z.; Ding, D.; Li, X.; Jia, Z.; Jiang, X.; Hu, Y. Covalently combining carbon nanotubes with anticancer agent: Preparation and antitumor activity. ACS Nano 2009, 22, 2740–2750. [Google Scholar] [CrossRef]
- Dintchevaa, T.; Carrocciod, S.; Arrigoab, R.; Bellaviaa, S.; Cristian Gambarottic, N. Carbon nanotubes-based nanohybrids for multifunctional nanocomposites. J. King Saud Univ. Sci. 2017, 29, 502–509. [Google Scholar] [CrossRef]
- Wang, C.; Li, W. Preparation, Characterization, and in Vitro and Vivo Antitumor Activity of Oridonin-Conjugated Multiwalled Carbon Nanotubes Functionalized with Carboxylic Group. J. Nanomater. 2016, 2016, 1–8. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, X.; Yang, M.; Li, X.; Mao, Y.; Guan, X.; Di, D.; Wang, S. Multi-stimuli responsive mesoporous carbon nano-platform gated by human serum albumin for cancer thermo-chemotherapy. Colloids Surfaces B Biointerfaces 2019, 184, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehra, N.K.; Jain, N.K. One platform comparison of estrone and folic acid anchored surface engineered MWCNTs for doxorubicin delivery. Mol. Pharm. 2015, 12, 630–643. [Google Scholar] [CrossRef]
- Douradinha, B.; Doolan, D.L. Harnessing immune responses against Plasmodium for rational vaccine design. Trends Parasitol. 2011, 6, 274–283. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konduru, N.V.; Tyurina, Y.Y.; Feng, W.; Basova, L.V.; Belikova, N.A.; Bayir, H.; Clark, K.; Rubin, M.; Stolz, D.; Vallhov, H.; et al. Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS ONE 2009, 4, e4398. [Google Scholar] [CrossRef]
- Pescatori, M.; Bedognetti, D.; Venturelli, E.; Ménard-Moyon, C.; Bernardini, C.; Muresu, E.; Piana, A.; Maida, G.; Manetti, R.; Sgarrella, F.; et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials 2013, 34, 4395–4403. [Google Scholar] [CrossRef] [Green Version]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Thordarson, P.; Gooding, J.J.; Ringer, S.P.; Braet, F. Carbon nanotubes for biological and biomedical applications. Nanotechnology 2007, 18, 1–12. [Google Scholar] [CrossRef]
- Atkinson, H.; Chalmers, R. Delivering the goods: Viral and non-viral gene therapy systems and the inherent limits on cargo DNA and internal sequences. Genetica 2010, 138, 485–498. [Google Scholar] [CrossRef]
- Md Saquib Hasnain, A.K.N. Carbon Nanotubes in Gene Delivery. In Springer Briefs in Applied Sciences and Technology; Springer: Hoboken, NJ, USA, 2019; pp. 75–87. ISBN 978-981-15-0909-4. [Google Scholar]
- Varkouhi, A.K.; Foillard, S.; Lammers, T.; Schiffelers, R.M.; Doris, E.; Hennink, W.E.; Storm, G. SiRNA delivery with functionalized carbon nanotubes. Int. J. Pharm. 2011, 416, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, M.; Tian, L.; Qiu, Y.; Yu, Q.; Wang, X.; Guo, R.; He, Q. Facile strategy by hyaluronic acid functional carbon dot-doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int. J. Pharm. 2020, 578, 119122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Niu, Y.; Zhu, J.; Gao, C.; Xu, Q.; He, Z.; Chen, D.; Xu, M.; Liu, Y. Tailor-made legumain/pH dual-responsive doxorubicin prodrug-embedded nanoparticles for efficient anticancer drug delivery and: In situ monitoring of drug release. Nanoscale 2020, 12, 2673–2685. [Google Scholar] [CrossRef]
- Shahabi, M.; Raissi, H. Payload delivery of anticancer drug Tegafur with the assistance of graphene oxide nanosheet during biomembrane penetration: Molecular dynamics simulation survey. Appl. Surf. Sci. 2020, 517, 146186. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 2018, 8, 1966–1984. [Google Scholar] [CrossRef]
- Oskoueian, A.; Amin Matori, K.; Bayat, S.; Oskoueian, E.; Ostovan, F.; Toozandehjani, M. Fabrication, Characterization, and Functionalization of Single-Walled Carbon Nanotube Conjugated with Tamoxifen and Its Anticancer Potential against Human Breast Cancer Cells. J. Nanomater. 2018, 2018. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, Z.; Behnam, M.A.; Emami, F.; Dehghanian, A.; Jamhiri, I. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes. Int. J. Nanomed. 2017, 12, 4509–4517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorica, C.; Mauro, N.; Pitarresi, G.; Scialabba, C.; Palumbo, F.S.; Giammona, G. Double-Network-Structured Graphene Oxide-Containing Nanogels as Photothermal Agents for the Treatment of Colorectal Cancer. Biomacromolecules 2017, 18, 1010–1018. [Google Scholar] [CrossRef]
- Ko, N.R.; Nafiujjaman, M.; Lee, J.S.; Lim, H.N.; Lee, Y.K.; Kwon, I.K. Graphene quantum dot-based theranostic agents for active targeting of breast cancer. RSC Adv. 2017, 7, 11420–11427. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Zhou, S.; Garcia, C.; Fan, L.; Zhou, J. PH-Responsive fluorescent graphene quantum dots for fluorescence-guided cancer surgery and diagnosis. Nanoscale 2017, 9, 4928–4933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eldridge, B.N.; Bernish, B.W.; Fahrenholtz, C.D.; Singh, R. Photothermal Therapy of Glioblastoma Multiforme Using Multiwalled Carbon Nanotubes Optimized for Diffusion in Extracellular Space. ACS Biomater. Sci. Eng. 2016, 2, 963–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, K.; Kumar, D.; Kiran, C.; Kumar, M.; Guru, S.K.; Kumar, P.; Arora, S.; Sharma, G.; Bhushan, S.; Katare, O.P. Conjugation of docetaxel with multiwalled carbon nanotubes and codelivery with piperine: Implications on pharmacokinetic profile and anticancer activity. Mol. Pharm. 2016, 13, 2423–2432. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K. Versatile photoluminescence from graphene and its derivatives. Carbon N. Y. 2015, 88, 86–112. [Google Scholar] [CrossRef]
- Pattnaik, S.; Swain, K.; Lin, Z. Graphene and graphene-based nanocomposites: Biomedical applications and biosafety. J. Mater. Chem. B 2016, 4, 7813–7831. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Khatun, Z.; Nafiujjaman, M.; Lee, D.G.; Lee, Y.K. Surface coating of graphene quantum dots using mussel-inspired polydopamine for biomedical optical imaging. ACS Appl. Mater. Interfaces 2013, 5, 8246–8253. [Google Scholar] [CrossRef]
- Zamani, M.; Rostami, M.; Aghajanzadeh, M.; Kheiri Manjili, H.; Rostamizadeh, K.; Danafar, H. Mesoporous titanium dioxide@ zinc oxide–graphene oxide nanocarriers for colon-specific drug delivery. J. Mater. Sci. 2018, 53, 1634–1645. [Google Scholar] [CrossRef]
- Lu, Y.J.; Lan, Y.H.; Chuang, C.C.; Lu, W.T.; Chan, L.Y.; Hsu, P.W.; Chen, J.P. Injectable thermo-sensitive chitosan hydrogel containing CPT-11-loaded EGFR-targeted graphene oxide and SLP2 shRNA for localized drug/gene delivery in glioblastoma therapy. Int. J. Mol. Sci. 2020, 21, 7111. [Google Scholar] [CrossRef]
- Ren, T.; Wang, Y.; Yu, Q.; Li, M. Synthesis of antimicrobial peptide-grafted graphene oxide nanosheets with high antimicrobial efficacy. Mater. Lett. 2019, 235, 42–45. [Google Scholar] [CrossRef]
- Khalili, R.; Zarrintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-graphene oxide/chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cui, X.; Xu, S.; Wang, X.; Chen, C. The nano-bio interaction and biomedical applications of carbon nanomaterials. Carbon N. Y. 2018, 138, 436–450. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Randviir, E.P.; Abo Dena, A.S.; Banks, C.E. An overview of recent applications of reduced graphene oxide as a basis of electroanalytical sensing platforms. Appl. Mater. Today 2018, 10, 218–226. [Google Scholar] [CrossRef]
- Badami, D.V. X-Ray studies of graphite formed by decomposing silicon carbide. Carbon N. Y. 1965, 3, 53–54. [Google Scholar] [CrossRef]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, K.; Feng, L.; Liu, Z. In vitro and in vivo behaviors of dextran functionalized graphene. Carbon2 2011, 49, 4040–4049. [Google Scholar] [CrossRef]
- Layek, R.K.; Uddin, M.E.; Kim, N.H.; Tak Lau, A.K.; Lee, J.H. Noncovalent functionalization of reduced graphene oxide with pluronic F127 and its nanocomposites with gum arabic. Compos. Part B Eng. 2017, 128, 155–163. [Google Scholar] [CrossRef]
- Fang, M.; Long, J.; Zhao, W.; Wang, L.; Chen, G. pH-responsive chitosan-mediated graphene dispersions. Langmuir 2010, 26, 16771–16774. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Liu, Z. Graphene based gene transfection. Nanoscale 2011, 3, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.; Näreoja, T.; Von Haartman, E.; Şen Karaman, D.; Burikov, S.A.; Dolenko, T.A.; Deguchi, T.; Mamaeva, V.; Hänninen, P.E.; Vlasov, I.I.; et al. Functionalization of graphene oxide nanostructures improves photoluminescence and facilitates their use as optical probes in preclinical imaging. Nanoscale 2015, 7, 10410–10420. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y.; Lee, B.S.; Park, S.; Yu, W.R. A facile route to mechanically robust graphene oxide fibers. RSC Adv. 2019, 9, 20248–20255. [Google Scholar] [CrossRef] [Green Version]
- Boran, G.; Tavakoli, S.; Dierking, I.; Kamali, A.R.; Ege, D. Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment. Sci. Rep. 2020, 10, 7827. [Google Scholar] [CrossRef] [PubMed]
- Islami, M.; Zarrabi, A.; Tada, S.; Kawamoto, M.; Isoshima, T.; Ito, Y. Controlled quercetin release from high-capacity-loading hyperbranched polyglycerol-functionalized graphene oxide. Int. J. Nanomed. 2018, 13, 6059–6071. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X.; et al. Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef] [PubMed]
- Cheon, Y.A.; Bae, J.H.; Chung, B.G. Reduced Graphene Oxide Nanosheet for Chemo-photothermal Therapy. Langmuir 2016, 32, 2731–2736. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.T.; Chen, C.H.; Chen, J.P. Intratumoral delivery of doxorubicin on folate-conjugated graphene oxide by in-situ forming thermo-sensitive hydrogel for breast cancer therapy. Nanomaterials 2017, 7, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, L.; Zhang, R.; Lu, J.; Zhao, C.; Deng, X.; Wu, Y. Mesoporous silica coated polydopamine functionalized reduced graphene oxide for synergistic targeted chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2017, 9, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Hasan, A.; Al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Sun, X.; Lao, J.; He, H.; Cheng, T.; Wang, M.; Wang, S.; Huang, F. Multifunctional graphene quantum dots for simultaneous targeted cellular imaging and drug delivery. Colloids Surfaces B Biointerfaces 2014, 122, 638–644. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Dowaidar, M.; Hällbrink, M.; Langel, Ü. Gene delivery using cell penetrating peptides-zeolitic imidazolate frameworks. Microporous Mesoporous Mater. 2020, 300, 110173. [Google Scholar] [CrossRef]
- Tian, B.; Wang, C.; Zhang, S.; Feng, L.; Liu, Z. Photothermally enhanced photodynamic therapy delivered by nano-graphene oxide. ACS Nano 2011, 5, 7000–7009. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zheng, S.; Zhang, S.; Wang, Y.; Yang, X.; Duan, H.; Huang, Y.; Chen, Y. Functionalized graphene oxide with hepatocyte targeting as anti-tumor drug and gene intracellular transporters. J. Nanosci. Nanotechnol. 2015, 15, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, D.; Tang, H.; Tan, L.; Xie, Q.; Zhang, Y.; Ma, M.; Yao, S. Polyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectors. ACS Appl. Mater. Interfaces 2014, 6, 8173–8183. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Rahmani, F.; Nouranian, S. Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems. J. Mater. Chem. B 2016, 4, 7441–7451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, L.; Li, X.; Jia, X.; Liu, L.; Zeng, J.; Guo, J.; Liu, P. Functionalized graphene oxide nanoparticles for cancer cell-specific delivery of antitumor drug. Bioconjug. Chem. 2015, 26, 128–136. [Google Scholar] [CrossRef]
- Farazi, R.; Vaezi, M.R.; Molaei, M.J.; Saeidifar, M.; Behnam-Ghader, A.A. Effect of pH and temperature on doxorubicin hydrochloride release from magnetite/graphene oxide nanocomposites. Mater. Today Proc. 2018, 5, 15726–15732. [Google Scholar] [CrossRef]
- Afzal, H.; Ikram, M.; Ali, S.; Shahzadi, A.; Aqeel, M.; Haider, A.; Imran, M.; Ali, S. Enhanced drug efficiency of doped ZnO-GO (graphene oxide) nanocomposites, a new gateway in drug delivery systems (DDSs). Mater. Res. Express 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Katuwavila, N.P.; Amarasekara, Y.; Jayaweera, V.; Rajaphaksha, C.; Gunasekara, C.; Perera, I.C.; Amaratunga, G.A.J.; Weerasinghe, L. Graphene Oxide–Based Nanocomposite for Sustained Release of Cephalexin. J. Pharm. Sci. 2020, 109, 1130–1135. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surfaces B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Nordin, N.I.; Dinshaw, I.J.; Lai, C.W.; Leo, B.F. Synergistic antibacterial actions of graphene oxide and antibiotics towards bacteria and the toxicological effects of graphene oxide on human epidermal keratinocytes. Eur. J. Pharm. Sci. 2020, 142, 105087. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Fattahi, A.; Tajkhorshid, E.; Nouranian, S. Molecular Insights into the Loading and Dynamics of Doxorubicin on PEGylated Graphene Oxide Nanocarriers. ACS Appl. Bio Mater. 2020, 3, 1354–1363. [Google Scholar] [CrossRef]
- Kumawat, M.K.; Thakur, M.; Gurung, R.B.; Srivastava, R. Graphene Quantum Dots for Cell Proliferation, Nucleus Imaging, and Photoluminescent Sensing Applications. Sci. Rep. 2017, 7, 15858. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, S.; Li, Y.; Li, X.; Zhu, J.; Fan, L.; Yang, S. Exceptionally High Payload of the IR780 Iodide on Folic Acid-Functionalized Graphene Quantum Dots for Targeted Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 22332–22341. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Gao, W.; Qiu, X.; Zhang, H.; Liu, L.; Liao, P.; Fu, W.; Luo, Y. Graphene quantum dots in biomedical applications: Recent advances and future challenges. Front. Lab. Med. 2017, 1, 192–199. [Google Scholar] [CrossRef]
- Pistone, A.; Iannazzo, D.; Ansari, S.; Milone, C.; Salamò, M.; Galvagno, S.; Cirmi, S.; Navarra, M. Tunable doxorubicin release from polymer-gated multiwalled carbon nanotubes. Int. J. Pharm. 2016, 515, 30–36. [Google Scholar] [CrossRef]
- Tian, Z.; Yao, X.; Ma, K.; Niu, X.; Grothe, J.; Xu, Q.; Liu, L.; Kaskel, S.; Zhu, Y. Metal-Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy. ACS Omega 2017, 2, 1249–1258. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.F.; Zhang, P.H.; Xi, Y.; Chen, J.J.; Li, L.L.; Zhu, J.J. Aptamer/Graphene Quantum Dots Nanocomposite Capped Fluorescent Mesoporous Silica Nanoparticles for Intracellular Drug Delivery and Real-Time Monitoring of Drug Release. Anal. Chem. 2015, 87, 11739–11745. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, F.; Zhao, C.; Lv, Y.; Ma, G.; Wei, W.; Tian, Z. Beyond a Carrier: Graphene Quantum Dots as a Probe for Programmatically Monitoring Anti-Cancer Drug Delivery, Release, and Response. ACS Appl. Mater. Interfaces 2017, 9, 27396–27401. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807–815. [Google Scholar] [CrossRef]
- Diederich, F.; Gómez-López, M. Supramolecular fullerene chemistry. Chem. Soc. Rev. 1999, 28, 263–277. [Google Scholar] [CrossRef]
- Xie, Q.; Pérez-Cordero, E.; Echegoyen, L. Electrochemical Detection of C606- and C706-: Enhanced Stability of Fullerides in Solution. J. Am. Chem. Soc. 1992, 114, 3978–3980. [Google Scholar] [CrossRef]
- Yan, W.; Seifermann, S.M.; Pierrat, P.; Bräse, S. Synthesis of highly functionalized C60 fullerene derivatives and their applications in material and life sciences. Org. Biomol. Chem. 2015, 13, 25–54. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, M.; Oh, I.K. Fullerenol-based electroactive artificial muscles utilizing biocompatible polyetherimide. ACS Nano 2011, 5, 2248–2256. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, H.; Tan, L. A novel fullerene-based drug delivery system delivering doxorubicin for potential lung cancer therapy. J. Nanosci. Nanotechnol. 2017, 17, 5147–5154. [Google Scholar] [CrossRef]
- Hazrati, M.K.; Bagheri, Z.; Bodaghi, A. Application of C30B15N15 heterofullerene in the isoniazid drug delivery: DFT studies. Phys. E Low Dimens. Syst. Nanostruct. 2017, 89, 72–76. [Google Scholar] [CrossRef]
- Tan, L.; Wu, T.; Tang, Z.W.; Xiao, J.Y.; Zhuo, R.X.; Shi, B.; Liu, C.J. Water-soluble photoluminescent fullerene capped mesoporous silica for pH-responsive drug delivery and bioimaging. Nanotechnology 2016, 27, 315104. [Google Scholar] [CrossRef]
- Ryan, J.J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerene Nanomaterials Inhibit the Allergic Response. J. Immunol. 2007, 179, 659–672. [Google Scholar] [CrossRef] [Green Version]
- Dellinger, A.; Zhou, Z.; Connor, J.; Madhankumar, A.; Pamujula, S.; Sayes, C.M.; Kepley, C.L. Application of fullerenes in nanomedicine: An update. Nanomedicine 2013, 8, 1191–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcroft, J.M.; Tsyboulski, D.A.; Hartman, K.B.; Zakharian, T.Y.; Marks, J.W.; Weisman, R.B.; Rosenblum, M.G.; Wilson, L.J. Fullerene (C60) immunoconjugates: Interaction of water-soluble C60 derivatives with the murine anti-gp240 melanoma antibody. Chem. Commun. 2006, 2006, 3004–3006. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Lee, J.Y.; Park, K.H.; Kim, W.; Lee, J.H.; Kang, H.J.; Hong, S.W.; Park, H.J.; Lee, S.; et al. Photosensitized Production of Singlet Oxygen via C60 Fullerene Covalently Attached to Functionalized Silica-coated Stainless-Steel Mesh: Remote Bacterial and Viral Inactivation. Appl. Catal. B Environ. 2020, 270, 118862. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Angus, J.C. Diamond synthesis by chemical vapor deposition: The early years. Diam. Relat. Mater. 2014, 49, 77–86. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Chang, B.M.; Chang, H.C. Nanodiamond-enabled biomedical imaging. Nanomedicine 2020, 15, 1599–1616. [Google Scholar] [CrossRef]
- Nunn, N.; Prabhakar, N.; Reineck, P.; Magidson, V.; Kamiya, E.; Heinz, W.F.; Torelli, M.D.; Rosenholm, J.; Zaitsev, A.; Shenderova, O. Brilliant blue, green, yellow, and red fluorescent diamond particles: Synthesis, characterization, and multiplex imaging demonstrations. Nanoscale 2019, 11, 11584–11595. [Google Scholar] [CrossRef]
- Prabhakar, N.; Rosenholm, J.M. Nanodiamonds for advanced optical bioimaging and beyond. Curr. Opin. Colloid Interface Sci. 2019, 39, 220–231. [Google Scholar] [CrossRef]
- Reineck, P.; Trindade, L.F.; Havlik, J.; Stursa, J.; Heffernan, A.; Elbourne, A.; Orth, A.; Capelli, M.; Cigler, P.; Simpson, D.A.; et al. Not All Fluorescent Nanodiamonds Are Created Equal: A Comparative Study. Part. Part. Syst. Charact. 2019, 36, 1900009. [Google Scholar] [CrossRef]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar] [CrossRef]
- Wilson, E.R.; Parker, L.M.; Orth, A.; Nunn, N.; Torelli, M.; Shenderova, O.; Gibson, B.C.; Reineck, P. The effect of particle size on nanodiamond fluorescence and colloidal properties in biological media. Nanotechnology 2019, 30, 385704. [Google Scholar] [CrossRef] [PubMed]
- Mengesha, A.E.; Youan, B.B.C. Nanodiamonds for drug delivery systems. In Diamond-Based Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2013; pp. 186–205. ISBN 9780857093400. [Google Scholar]
- Rehor, I.; Slegerova, J.; Kucka, J.; Proks, V.; Petrakova, V.; Adam, M.-P.; Treussart, F.; Turner, S.; Bals, S.; Sacha, P.; et al. Fluorescent Nanodiamonds Embedded in Biocompatible Translucent Shells. Small 2014, 10, 1106–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Haartman, E.; Jiang, H.; Khomich, A.A.; Zhang, J.; Burikov, S.A.; Dolenko, T.A.; Ruokolainen, J.; Gu, H.; Shenderova, O.A.; Vlasov, I.I.; et al. Core-shell designs of photoluminescent nanodiamonds with porous silica coatings for bioimaging and drug delivery I: Fabrication. J. Mater. Chem. B 2013, 1, 2358–2366. [Google Scholar] [CrossRef]
- Rosenholm, J.M.; Vlasov, I.I.; Burikov, S.A.; Dolenko, T.A.; Shenderova, O.A. Nanodiamond-based composite structures for biomedical imaging and drug delivery. J. Nanosci. Nanotechnol. 2015, 15, 959–971. [Google Scholar] [CrossRef]
- Roy, U.; Drozd, V.; Durygin, A.; Rodriguez, J.; Barber, P.; Atluri, V.; Liu, X.; Voss, T.G.; Saxena, S.; Nair, M. Characterization of Nanodiamond-based anti-HIV drug Delivery to the Brain. Sci. Rep. 2018, 8, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.C.; González, C.H.; Miller, B.S.; Edgington, R.J.; Ferretti, P.; Jackman, R.B. Surface functionalisation of nanodiamonds for human neural stem cell adhesion and proliferation. Sci. Rep. 2017, 7, 7307. [Google Scholar] [CrossRef] [PubMed]
- Edgington, R.; Jackman, R.B. Neuron growth on nanodiamond. In Nanodiamond; Royal Society of Chemistry: London, UK, 2014; pp. 195–220. ISBN 9781849736398. [Google Scholar]
- Bartolome, J.P.; Fragoso, A. Preparation and characterization of carbon nano-onions by nanodiamond annealing and functionalization by radio-frequency Ar/O2 plasma. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 327–334. [Google Scholar] [CrossRef]
- Palkar, A.; Melin, F.; Cardona, C.M.; Elliott, B.; Naskar, A.K.; Edie, D.D.; Kumbhar, A.; Echegoyen, L. Reactivity differences between Carbon Nano Onions (CNOs) prepared by different methods. Chem. Asian J. 2007, 2, 625–633. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef]
- Sano, N.; Wang, H.; Alexandrou, I.; Chhowalla, M.; Teo, K.B.K.; Amaratunga, G.A.J.; Iimura, K. Properties of carbon onions produced by an arc discharge in water. J. Appl. Phys. 2002, 92, 2783–2788. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.L.; Chuvilin, A.L.; Butenko, Y.V.; Mal’kov, I.Y.; Titov, V.M. Onion-like carbon from ultra-disperse diamond. Chem. Phys. Lett. 1994, 222, 343–348. [Google Scholar] [CrossRef]
- Mykhailiv, O.; Lapinski, A.; Molina-Ontoria, A.; Regulska, E.; Echegoyen, L.; Dubis, A.T.; Plonska-Brzezinska, M.E. Influence of the Synthetic Conditions on the Structural and Electrochemical Properties of Carbon Nano-Onions. ChemPhysChem 2015, 16, 2182–2191. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Rummeli, M.H.; Gemming, T.; Lange, H.; Huczko, A. Catalyst-free synthesis of onion-like carbon nanoparticles. Xinxing Tan Cailiao/New Carbon Mater. 2010, 25, 1–8. [Google Scholar] [CrossRef]
- Camisasca, A.; Giordani, S. Carbon nano-onions in biomedical applications: Promising theranostic agents. Inorg. Chim. Acta 2017, 468, 67–76. [Google Scholar] [CrossRef]
- Bartelmess, J.; Giordani, S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein J. Nanotechnol. 2014, 5, 1980–1998. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; González-Ortiz, A.; Romo, I.L.; Barrera, E.V. Development of functionalized carbon nano-onions reinforced zein protein hydrogel interfaces for controlled drug release. Pharmaceutics 2019, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- D’Amora, M.; Camisasca, A.; Boarino, A.; Giordani, S.; Arpicco, S. Supramolecular functionalization of carbon nano-onions with hyaluronic acid-phospholipid conjugates for selective targeting of cancer cells. Colloids Surfaces B Biointerfaces 2020, 188, 110779. [Google Scholar] [CrossRef] [PubMed]
- Bobrowska, D.M.; Czyrko, J.; Brzezinski, K.; Echegoyen, L.; Plonska-Brzezinska, M.E. Carbon nano-onion composites: Physicochemical characteristics and biological activity. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 185–192. [Google Scholar] [CrossRef]
- Babar, D.G.; Pakhira, B.; Sarkar, S. DNA–carbon nano onion aggregate: Triangle, hexagon, six-petal flower to dead-end network. Appl. Nanosci. 2017, 7, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, K.M.; Bhati, A.; Singh, A.; Gupta, N.R.; Verma, S.; Sarkar, S.; Sonkar, S.K. From the traditional way of pyrolysis to tunable photoluminescent water soluble carbon nano-onions for cell imaging and selective sensing of glucose. RSC Adv. 2016, 6, 37319–37329. [Google Scholar] [CrossRef]
- Pakhira, B.; Ghosh, M.; Allam, A.; Sarkar, S. Carbon nano onions cross the blood brain barrier†. RSC Adv. 2016, 6, 29779–29782. [Google Scholar] [CrossRef]
- Mamidi, N.; Zuníga, A.E.; Villela-Castrejón, J. Engineering and evaluation of forcespun functionalized carbon nano-onions reinforced poly (ε-caprolactone) composite nanofibers for pH-responsive drug release. Mater. Sci. Eng. C 2020, 112, 110928. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Villela Castrejón, J.; González-Ortiz, A. Rational design and engineering of carbon nano-onions reinforced natural protein nanocomposite hydrogels for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103696. [Google Scholar] [CrossRef]
- Tovar, C.D.G.; Castro, J.I.; Valencia, C.H.; Porras, D.P.N.; Hernandez, J.H.M.; Valencia, M.E.; Velásquez, J.D.; Chaur, M.N. Preparation of chitosan/poly(Vinyl alcohol) nanocomposite films incorporated with oxidized carbon nano-onions (multi-layer fullerenes) for tissue-engineering applications. Biomolecules 2019, 9, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lettieri, S.; Camisasca, A.; D’Amora, M.; Diaspro, A.; Uchida, T.; Nakajima, Y.; Yanagisawa, K.; Maekawa, T.; Giordani, S. Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Adv. 2017, 7, 45676–45681. [Google Scholar] [CrossRef] [Green Version]
- Sok, V.; Fragoso, A. Preparation and characterization of alkaline phosphatase, horseradish peroxidase, and glucose oxidase conjugates with carboxylated carbon nano-onions. Prep. Biochem. Biotechnol. 2018, 48, 136–143. [Google Scholar] [CrossRef]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, A.M.; Kurtycz, P.; Olszyna, A.R. Recent advances in graphene family materials toxicity investigations. J. Nanoparticle Res. 2012, 14, 1320. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Allegri, M.; Perivoliotis, D.K.; Bianchi, M.G.; Chiu, M.; Pagliaro, A.; Koklioti, M.A.; Trompeta, A.F.A.; Bergamaschi, E.; Bussolati, O.; Charitidis, C.A. Toxicity determinants of multi-walled carbon nanotubes: The relationship between functionalization and agglomeration. Toxicol. Rep. 2016, 3, 230–243. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef] [Green Version]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, R.; Ilyas, A.M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon nanotubes in biomedical applications: Factors, mechanisms, and remedies of toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.W.; Guy, R.C. Nanotoxicology. Encycl. Toxicol. Third Ed. 2014, 61, 727–738. [Google Scholar] [CrossRef]
- Wang, J.; Cao, S.; Ding, Y.; Ma, F.; Lu, W.; Sun, M. Theoretical investigations of optical origins of fluorescent graphene quantum dots. Sci. Rep. 2016, 6, 24850. [Google Scholar] [CrossRef]
- Nam, C.W.; Kang, S.J.; Kang, Y.K.; Kwak, M.K. Cell growth inhibition and apoptosis by SDS-solubilized single-walled carbon nanotubes in normal rat kidney epithelial cells. Arch. Pharmacal Res. 2011, 34, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, T.; Kim, Y.S.; Choi, H.S.; Lim, H.J.; Yang, S.J.; Park, C.R. Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers. Carbon N. Y. 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Praena, D.G.; Pichardo, S.; Sánchez, E.; Grilo, A.; Cameán, A.M.; Jos, A. Influence of carboxylic acid functionalization on the cytotoxic effects induced by single wall carbon nanotubes on human endothelial cells (HUVEC). Toxicol. In Vitro 2011, 25, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Kim, Y.A.; Hayashi, T.; Takeuchi, K.; Fujii, C.; Kuroiwa, N.; Koyama, H.; Tsukahara, T.; Endo, M. In vivo immunological toxicity in mice of carbon nanotubes with impurities. Carbon N. Y. 2009, 47, 1365–1372. [Google Scholar] [CrossRef] [Green Version]
- Aldieri, E.; Fenoglio, I.; Cesano, F.; Gazzano, E.; Gulino, G.; Scarano, D.; Attanasio, A.; Mazzucco, G.; Ghigo, D.; Fubini, B. The role of iron impurities in the toxic effects exerted by short multiwalled carbon nanotubes (MWCNT) in murine alveolar macrophages. J. Toxicol. Environ. Heal. Part A Curr. Issues 2013, 76, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced cytotoxicity of graphene nanosheets mediated by blood-protein coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef]
- Tian, X.; Xiao, B.B.; Wu, A.; Yu, L.; Zhou, J.; Wang, Y.; Wang, N.; Guan, H.; Shang, Z.F. Hydroxylated-graphene quantum dots induce cells senescence in both p53-dependent and -independent manner. Toxicol. Res. 2016, 5, 1639–1648. [Google Scholar] [CrossRef] [Green Version]
- Nurunnabi, M.; Khatun, Z.; Huh, K.M.; Park, S.Y.; Lee, D.Y.; Cho, K.J.; Lee, Y.K. In Vivo biodistribution and toxicology of carboxylated graphene quantum dots. ACS Nano 2013, 7, 6858–6867. [Google Scholar] [CrossRef] [PubMed]
- Garriga, R.; Herrero-Continente, T.; Palos, M.; Cebolla, V.L.; Osada, J.; Muñoz, E.; Rodríguez-Yoldi, M.J. Toxicity of carbon nanomaterials and their potential application as drug delivery systems: In vitro studies in caco-2 and mcf-7 cell lines. Nanomaterials 2020, 10, 1617. [Google Scholar] [CrossRef]
- Dasari Shareena, T.P.; McShan, D.; Dasmahapatra, A.K.; Tchounwou, P.B. A Review on Graphene-Based Nanomaterials in Biomedical Applications and Risks in Environment and Health. Nano Micro Lett. 2018, 10, 53. [Google Scholar] [CrossRef]
- Chauhan, S.; Jain, N.; Nagaich, U. Nanodiamonds with powerful ability for drug delivery and biomedical applications: Recent updates on in vivo study and patents. J. Pharm. Anal. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mochalin, V.N.; Neitzel, I.; Knoke, I.Y.; Han, J.; Klug, C.A.; Zhou, J.G.; Lelkes, P.I.; Gogotsi, Y. Fluorescent PLLA–nanodiamond composites for bone tissue engineering. Biomaterials 2011, 32, 87–94. [Google Scholar] [CrossRef]
- Schrand, A.M. Safety of Nanoparticles: From Manufacturing to Medical Applications; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-0-387-78608-7. [Google Scholar]
- Yuan, Y.; Wang, X.; Jia, G.; Liu, J.H.; Wang, T.; Gu, Y.; Yang, S.T.; Zhen, S.; Wang, H.; Liu, Y. Pulmonary toxicity and translocation of nanodiamonds in mice. Diam. Relat. Mater. 2010, 19, 291–299. [Google Scholar] [CrossRef]
- Mohan, N.; Chen, C.S.; Hsieh, H.H.; Wu, Y.C.; Chang, H.C. In vivo imaging and toxicity assessments of fluorescent nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef]
- Chow, E.K.; Zhang, X.Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond therapeutic delivery agents mediate enhanced chemoresistant tumor treatment. Sci. Transl. Med. 2011, 3, 73ra21. [Google Scholar] [CrossRef]
- Lacerda, L.; Bianco, A.; Prato, M.; Kostarelos, K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Adv. Drug Deliv. Rev. 2006, 58, 1460–1470. [Google Scholar] [CrossRef]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon nanotubes: A review of their properties in relation to pulmonary toxicology and workplace safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef] [Green Version]
- Ali-Boucetta, H.; Nunes, A.; Sainz, R.; Herrero, M.A.; Tian, B.; Prato, M.; Bianco, A.; Kostarelos, K. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew. Chem. Int. Ed. 2013. [Google Scholar] [CrossRef]
- Rungrotmongkol, T.; Arsawang, U.; Iamsamai, C.; Vongachariya, A.; Dubas, S.T.; Ruktanonchai, U.; Soottitantawat, A.; Hannongbua, S. Increased dispersion and solubility of carbon nanotubes noncovalently modified by the polysaccharide biopolymer, chitosan: MD simulations. Chem. Phys. Lett. 2011, 507, 134–137. [Google Scholar] [CrossRef]
- Kam, N.W.S.; Liu, Z.; Dai, H. Functionalization of carbon nanotubes via cleavable disulfide bonds for efficient intracellular delivery of siRNA and potent gene silencing. J. Am. Chem. Soc. 2005, 127, 12492–12493. [Google Scholar] [CrossRef]
- Russier, J.; León, V.; Orecchioni, M.; Hirata, E.; Virdis, P.; Fozza, C.; Sgarrella, F.; Cuniberti, G.; Prato, M.; Vázquez, E.; et al. Few-Layer Graphene Kills Selectively Tumor Cells from Myelomonocytic Leukemia Patients. Angew. Chem. Int. Ed. 2017, 56, 3014–3019. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Ferrari, M. The success of nanomedicine. Nano Today 2020, 31, 100853. [Google Scholar] [CrossRef]











| S. No. | Carbon-Based Nanomaterials | Advantages | Limitations in Biomedical Applications |
|---|---|---|---|
| 1. | Carbon nanotubes | Has good mechanical strength, aspect ratio, conductivity, and chemical stability. Offers tunable physical properties (e.g., diameter, length, single-walled vs. multi-walled, surface functionalization, and chirality), biocompatibility, and high surface area. | Lack of solubility in aqueous media, non-homogenous in size (diameter and length), and possibilities of metallic impurities. Pristine CNTs being a lightweight powder may enter into the respiratory tract. |
| 2. | Graphene/graphene oxide/graphene quantum dots | Offers excellent electrical, optical, and thermal properties. The two-dimensional atomic sheet structure of graphene enables more diverse electronic characteristics than CNTs. | Colloidal instability, lack of reproducibility, limited synthetic control, poor chemical stability in the biological environment, susceptibility to the oxidative environment. |
| 3. | Fullerenes | Peculiar photoelectrochemical properties, the possibility of surface modification, and superconductivity. | Low aqueous solubility, accumulation in cell membranes, susceptible to degradation in the presence of light and oxygen, susceptible to deactivation process such as quenching. |
| 4. | Nanodiamonds | Fluorescence and photoluminescence, biocompatible, smaller size compared to other CNMs, hard, corrosion-resistant, chemical inertness, high electrical resistance, and optical transparency. | Difficult to manufacture via covalent manner, tedious to remove toxic organic solvents while fabrication, abrupt drug release, and tendency to aggregate. |
| 5. | Carbon nano-onions | Unique electronic and structural properties, including the ability to accept electrons reversibly, a high surface-area to volume ratio, and broad absorption bands. | Hydrophobic, low biocompatibility, aggregation, prone to oxidation, low surface chemical reactivity. |
| S. No. | Indication | Drug/Vaccines/Genes | CNT Functionalization | Results | References |
|---|---|---|---|---|---|
| 1. | Cell proliferation | Tissue engineering | Hydrogels of PEG-CNTs | Pristine CNTs and PEG-CNT hydrogels enhanced the cytocompatibility, viability, and proliferation of L29 fibroblasts. | [39] |
| 2. | Cancer | Gemcitabine–Lentinan | MWCNTs/Gemcitabine (Ge)/Lentinan-Le | MWCNTs-Ge-Le showed augmented chemo and near IR-photothermal synergistic antitumor activity. | [40] |
| 3. | Ischemic brain tissue | Dexamethasone | PEGylated vertically aligned MWCNTs | Low cytotoxicity was observed on the PC-12 cell line by the MWCNTs. | [41] |
| 4. | Bladder cancer | Epirubicin (Epi) | Magnetic MWCNTs-Epi | Externally magnetic guided MWCNTs showed enhanced sustained release, prolonged retention behavior, and better antitumor activity than the free epirubicin both in vitro and in vivo. | [42] |
| 5. | Antileishmanial | Cisplatin (Ci) | Ci-SWCNTs, Ci-MWCNTs | Ci-MWCNTs showed potent in vitro antileishmanial activity at low concentrations on Leishmania. major. | [43] |
| 6. | Antibacterial activity | Curcumin | Glucose-modified calcium alginate single-walled carbon nanotubes (CA/SWCNT-Gl) | CA/SWCNT-Gl nanocomposite showed better antibacterial activity against Bacillus cereus and Escherichia coli. | [44] |
| 7. | Cancer therapy | Doxorubicin (DOX) | N-isopropyl acrylamide carbon nanotubes loaded with DOX | Smaller polymer chain length resulted in more hydrogen bonding with the drug, five mer N-isopropyl acrylamide augmented DOX loading. | [45] |
| 8. | Cancer therapy | Doxorubicin | A pH-sensitive conjugate of DOX-loaded SWCNTs and MWCNTs | Bonds between DOX and MWCNTs were stronger, which resulted in controlled drug release in cancer tissues in comparison to SWCNTs. | [46] |
| 9. | Genetic engineering | Plastid genome | Chitosan complexed SWCNTs utilizing the lipid exchange envelope penetration mechanism | Chitosan–SWCNTs selectively delivered plasmid DNA to the chloroplast in Eruca sativa, Nasturtum officinale, Nicotiana tabacum, and Soiacia oleracea, and isolated Arabidopsos thaliana mesophyll protoplasts. | [47] |
| 10. | Peptide delivery | GS-protein | The casing of SWNTs with polycationic and amphiphilic peptides [H-(-Lys-Trp-Lys-Gly-)7-OH] | Seven times more uptake in comparison with the SWCNT–peptide composite without PEGylation. | [48] |
| S. No. | Carbon-Based Nanovectors | In Vitro | Indication | Reference |
|---|---|---|---|---|
| 1. | Hyaluronic acid-modified carbon dots | 4T1 cells | In vivo anti-tumor activity | [103] |
| 2. | Poly(ethylene glycol)-block-poly(β-benzyl-L-aspartate) (PEG-b-PBLA) polymers, octadecyl amine-p(Asp-API)10 (OAPI) polymers, and legumain-cleavable linker containing doxorubicin (DOX)-carbon quantum dots conjugations (CDs-C9-AANL-DOX) | Tumor cells | In vivo anti-tumor activity | [104] |
| 3. | Tegafur loaded graphene oxide nanosheet | Molecular dynamics simulation survey for drug release across the cell membrane | Antitumor activity | [105] |
| 4. | Epirubicin loaded multi-walled CNTS | T24 and 5637 cells | Bladder cancer | [42] |
| 5. | Single-walled CNTs | 4T1 cells | Chemo-photothermal therapy | [106] |
| 6. | Single-walled CNTs | Human breast cancer cell (MCF-7) | - | [107] |
| 7. | Single-walled CNTs | A549 and NIH 3T3 cells | - | [84] |
| 8. | Multi-walled CNTs | HeLa cells | Photothermal therapy | [108] |
| 9. | Double network structured GO | HCT116 cells | Chemo-photothermal | [109] |
| 10. | Graphene QDs | BT-474, MCF-7 cells | - | [110] |
| 11. | Graphene QDs | PANC-1, A-549, HepG2 | - | [111] |
| 12. | PEG functionalized multi-walled CNTS | U87, U373MG, NHA | Brain tumor therapy | [112] |
| 13. | Multi-walled CNTs | MCF-7 and MDA-MB-231 human breast cancer cells/rats | - | [113] |
| S. No. | Graphene Derivative | Therapeutic Agents | Application and Outcomes | References |
|---|---|---|---|---|
| 1. | GO | Zoledronic acid | Bone marrow-derived mesenchymal stem cells (BM-MSC), and Michigan Cancer Foundation-7 (MCF-7) breast cancer cells | [133] |
| 2. | Pristine graphene and graphene oxide | Doxorubicin | In vitro: pH simulation | [145] |
| 3. | Graphene oxide | Doxorubicin | In vitro: drug release | [146] |
| 4. | GO-FA-AuNPs | Doxorubicin | Significant in vivo tumor reversion in solid tumor model in Balb/c mice | [147] |
| 5. | GO nanosheets doped into ZnO NPs | Doxorubicin | GO-doped ZnO NPs displayed higher drug loading efficiency of 89% in comparison to 82% of ZnO. The developed system enhanced the dissolution of the drug. | [148] |
| 6. | PEG-functionalized GO | Cephalexin | In vitro, CEF release exhibited burst release followed by sustained release over the 96-h period with a cumulative release of 80%. MIC values stated dose and time-dependent antibacterial activity for GO-PEG-CEF against both Gram positive and Gram negative bacteria. | [149] |
| 7. | GO-PVP | Quercetin and gefitinib | Dual drug loaded GO-PVP nano-vehicles showed higher drug loading, and cancer cell cytotoxicity was more in contrast to an individual GO-PVP system in PA-1 ovarian cancer cells and compared to their effects on IOSE-364 ovarian epithelial cells. | [150] |
| 8. | GO | Ampicillin, chloramphenicol and tetracycline | GO potential as an antibacterial along with antibiotic drugs displayed synergistic effects against S. aureus, E. coli, E. faecalis, and P. aeruginosa and the toxicological effects of GO toward human epidermal keratinocytes (HaCaT). | [151] |
| 9. | PEGylated GO | Doxorubicin | PEGylation of the GO efficiently augmented the average water density around the nanocarrier, which acts as a barricade, leading to the DOX migration to the solvated PEG-free part of the GO surface. The computational results exhibited the fact that increasing the PEG chain length aids DOX loading on the nanocarrier. | [152] |
| S. No. | Delivery System | Application | Results | Reference |
|---|---|---|---|---|
| 1. | Polycaprolactone/f-CNO nanocomposite fiber | Anticancer drug delivery | DOX release from PCL/f-CNO nanocomposite fiber was pH-dependent. F-CNOs augmented the mechanical strength, hydrophobicity, and biocompatibility of the PCL nanofibers. | [203] |
| 2. | f-CNO-reinforced zein hydrogels | Anticancer drug delivery | f-CNOs improved the mechanical strength of zein hydrogels. The delivery system exhibited good cytocompatibility against the osteoblast cell line. A pH-responsive sustained drug release over 15 days was observed. | [197] |
| 3. | f-CNO/gelatin composite hydrogels | Anticancer drug delivery | 5-FU/f-CNO/Gelatin hydrogels exhibited augmented tensile strength in comparison to pristine gelatin hydrogels. A sustained release of 5-FU for over 15 days was observed, which indicated possible prospects in cartilage tissue engineering and drug delivery. | [204] |
| 4. | Ox-CNO-loaded chitosan polyvinyl alcohol (CS/PVA/oc-CNO) nanocomposite film | Tissue engineering application | Ox-CNO enhanced the stability of the CS/PVA/ox-CNO scaffold. The nanocomposite film displayed no allergic response or pus formation in Wistar rats after subdermal implantation of the scaffold. The CS/PVA/oc-CNO scaffold exhibited tissue regeneration capability. | [205] |
| 5. | Pristine CNOs (p-CNOs), ox-CNOs, far-red fluorescent-CNOs | Cellular imaging application | Ox-CNOS and Fluo-CNOs exhibited excellent cytocompatibility against MCF-7 and HeLa cells. Far-red fluorescent images indicated the internalization of fluo-CNOs by the MCF-7 cells, conforming that fluo-CNOs can be utilized as a high-resolution cellular imaging agent as an alternative for organic dyes. | [206] |
| 6. | DNA sensor composed of glassy carbon electrode (GCE)/pristine-CNO | Sensing biomolecular interaction | The GCE/CNO nanocomposite DNA sensor sensed the human papillomavirus oncogene DNA sequence via amperometric detection. Modification via electrochemical grafting of the GCE/CNO surface with two different diazonium salts (4-aminophenylacetic acid) PAA and (4-aminophenylmaleimide) PM yielded GCE/CNO/PAA and GCE/CNO/PM nanocomposite. Both surfaces supported the attachment of thiolated or biotinylated short recognition DNA sequences (DNA probes). The large surface area and the enhanced electron transfer properties of this analytical sensor helped in the sensing of biomolecular interactions. | [188] |
| 7. | CNO-based catalytic biosensor | Nanobiocatalyst for biosensing application | CNO-based catalytic biosensor (CNO/enzyme) conjugate retained optimum pH and temperature, displayed stability over a longer duration at 37 °C. The CNO catalytic biosensor efficiently detected the immobilization of various enzymes such as alkaline phosphatase, horseradish peroxidase (HRP), and glucose oxidase (≈0.5 mg of enzyme per mg of CNOs). | [207] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahor, A.; Singh, P.P.; Bharadwaj, P.; Sharma, N.; Yadav, S.; Rosenholm, J.M.; Bansal, K.K. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C 2021, 7, 19. https://doi.org/10.3390/c7010019
Mahor A, Singh PP, Bharadwaj P, Sharma N, Yadav S, Rosenholm JM, Bansal KK. Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C. 2021; 7(1):19. https://doi.org/10.3390/c7010019
Chicago/Turabian StyleMahor, Alok, Prem Prakash Singh, Peeyush Bharadwaj, Neeraj Sharma, Surabhi Yadav, Jessica M. Rosenholm, and Kuldeep K. Bansal. 2021. "Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology" C 7, no. 1: 19. https://doi.org/10.3390/c7010019
APA StyleMahor, A., Singh, P. P., Bharadwaj, P., Sharma, N., Yadav, S., Rosenholm, J. M., & Bansal, K. K. (2021). Carbon-Based Nanomaterials for Delivery of Biologicals and Therapeutics: A Cutting-Edge Technology. C, 7(1), 19. https://doi.org/10.3390/c7010019