Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy
Abstract
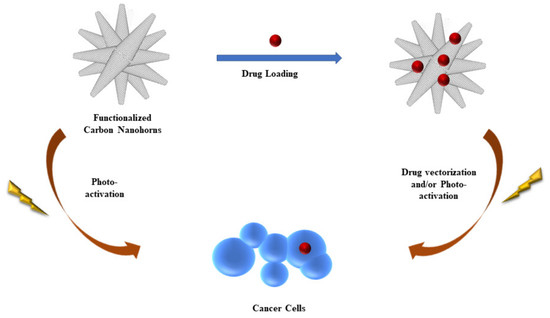
:1. Introduction
2. CNH: A Bridge between Carbon Nanotubes and Fullerenes
3. Functionalization of CNH for Biomedical Applications
3.1. Non-Covalent Functionalization of CNH for Application in Oncology
3.2. Covalent Functionalization of CNH for Application in Oncology
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Current concepts: Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stylianopoulos, T.; Jain, R.K. Design considerations for nanotherapeutics in oncology. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- International Agency for Research on Cancer. Cancer Tomorrow; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Norouzi, M.; Amerian, M.; Atyabi, F. Clinical applications of nanomedicine in cancer therapy. Drug Discov. Today 2020, 25, 107–125. [Google Scholar] [CrossRef]
- Lytton-Jean, A.K.R.; Kauffman, K.J.; Kaczmarek, J.C.; Langer, R. Cancer nanotherapeutics in clinical trials. In Cancer Treatment and Research; Springer: Cham, Switzerland, 2015; Volume 166, pp. 293–322. [Google Scholar]
- Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Targeted nanotechnologies for cancer intervention: A patent review (2010–2016). Expert Opin. Ther. Pat. 2017, 27, 1005–1019. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Peters, G.J.; Ossendorp, F.; Cruz, L.J. The potential of multi-compound nanoparticles to bypass drug resistance in cancer. Cancer Chemother. Pharmacol. 2017, 80, 881–894. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Zhuang, Q.; Ji, T.; Zhang, Y.; Li, C.; Wang, Y.; Li, H.; Jia, H.; Liu, Y.; Du, L. Multi-functionalized chitosan nanoparticles for enhanced chemotherapy in lung cancer. Carbohydr. Polym. 2018, 195, 311–320. [Google Scholar] [CrossRef]
- Weeks, J.C.; Catalano, P.J.; Cronin, A.; Finkelman, M.D.; Mack, J.W.; Keating, N.L.; Schrag, D. Patients’ expectations about effects of chemotherapy for advanced cancer. N. Engl. J. Med. 2012, 367, 1616–1625. [Google Scholar] [CrossRef] [Green Version]
- Peitzsch, C.; Cojoc, M.; Hein, L.; Kurth, I.; Mäbert, K.; Trautmann, F.; Klink, B.; Schröck, E.; Wirth, M.P.; Krause, M.; et al. An Epigenetic Reprogramming Strategy to Resensitize Radioresistant Prostate Cancer Cells. Cancer Res. 2016, 76, 2637–2651. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Fan, C.Q.; Dong, H.; Wang, S.M.; Yang, X.C.; Yang, S.M. Current applications and future prospects of nanomaterials in tumor therapy. Int. J. Nanomed. 2017, 12, 1815–1825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsaab, H.O.; Alghamdi, M.S.; Alotaibi, A.S.; Alzhrani, R.; Alwuthaynani, F.; Althobaiti, Y.S.; Almalki, A.H.; Sau, S.; Iyer, A.K. Progress in clinical trials of photodynamic therapy for solid tumors and the role of nanomedicine. Cancers 2020, 12, 2793. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, Y.; Huo, M.; Zhang, J.; Li, Y. Sequential catalytic nanomedicine augments synergistic chemodrug and chemodynamic cancer therapy. Nanoscale Horiz. 2019, 4, 890–901. [Google Scholar] [CrossRef]
- Bhise, K.; Sau, S.; Alsaab, H.; Kashaw, S.K.; Tekade, R.K.; Iyer, A.K. Nanomedicine for cancer diagnosis and therapy: Advancement, success and structure-activity relationship. Ther. Deliv. 2017, 8, 1003–1018. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorgan. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 147–166. [Google Scholar] [CrossRef]
- Lee, D.E.; Koo, H.; Sun, I.C.; Ryu, J.H.; Kim, K.; Kwon, I.C. Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 2012, 41, 2656–2672. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in nanomedicine: Approved and investigational nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Rafiee, S.D.; Reis, C.; Milosevic, A.; Moore, T.L.; Balog, S.; Rothen-Rutishauser, B.; Ruegg, C.; Petri-Fink, A. A rational and iterative process for targeted nanoparticle design and validation. Colloids Surf. B Biointerfaces 2018, 171, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Shibu, E.S.; Hamada, M.; Murase, N.; Biju, V. Nanomaterials formulations for photothermal and photodynamic therapy of cancer. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 53–72. [Google Scholar] [CrossRef]
- Yamashita, T.; Yamashita, K.; Nabeshi, H.; Yoshikawa, T.; Yoshioka, Y.; Tsunoda, S.I.; Tsutsumi, Y. Carbon nanomaterials: Efficacy and safety for nanomedicine. Materials 2012, 5, 350–363. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, D.; Bhushan, B. Friction, wear and mechanical behavior of nano-objects on the nanoscale. Mater. Sci. Eng. R Rep. 2015, 95, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Kostarelos, K.; Prato, M. Opportunities and challenges of carbon-based nanomaterials for cancer therapy. Expert Opin. Drug Deliv. 2008, 5, 331–342. [Google Scholar] [CrossRef]
- Loh, K.P.; Ho, D.; Chiu, G.N.C.; Leong, D.T.; Pastorin, G.; Chow, E.K.H. Clinical Applications of Carbon Nanomaterials in Diagnostics and Therapy. Adv. Mater. 2018, 30, 1802368. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [Green Version]
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Mehra, N.K.; Jain, A.K.; Nahar, M. Carbon nanomaterials in oncology: An expanding horizon. Drug Discov. Today 2018, 23, 1016–1025. [Google Scholar] [CrossRef]
- MacDonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Sawdon, A.; Weydemeyer, E.; Peng, C.A. Tumor photothermolysis: Using carbon nanomaterials for cancer therapy. Eur. J. Nanomed. 2013, 5, 131–140. [Google Scholar] [CrossRef]
- Augustine, S.; Singh, J.; Srivastava, M.; Sharma, M.; Das, A.; Malhotra, B.D. Recent advances in carbon based nanosystems for cancer theranostics. Biomater. Sci. 2017, 5, 901–952. [Google Scholar] [CrossRef] [PubMed]
- Biagiotti, G.; Fedeli, S.; Tuci, G.; Luconi, L.; Giambastiani, G.; Brandi, A.; Pisaneschi, F.; Cicchi, S.; Paoli, P. Combined therapies with nanostructured carbon materials: There is room still available at the bottom. J. Mater. Chem. B 2018, 6, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Curcio, M.; Cirillo, G.; Spataro, T.; Vittorio, O.; Picci, N.; Hampel, S.; Iemma, F.; Nicoletta, F.P. Recent advances in the synthesis and biomedical applications of nanocomposite hydrogels. Pharmaceutics 2015, 7, 413–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H.J. Carbon materials for drug delivery & cancer therapy. Mater. Today 2011, 14, 316–323. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-based nanomaterials/allotropes: A glimpse of their synthesis, properties and some applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Vedhanarayanan, B.; Praveen, V.K.; Das, G.; Ajayaghosh, A. Hybrid materials of 1D and 2D carbon allotropes and synthetic π-systems. NPG Asia Mater. 2018, 10, 107–126. [Google Scholar] [CrossRef]
- Wu, Y.F.; Wu, H.C.; Kuan, C.H.; Lin, C.J.; Wang, L.W.; Chang, C.W.; Wang, T.W. Multi-functionalized carbon dots as theranostic nanoagent for gene delivery in lung cancer therapy. Sci. Rep. 2016, 6, 21170. [Google Scholar] [CrossRef]
- Lin, H.S.; Matsuo, Y. Functionalization of [60] fullerene through fullerene cation intermediates. Chem. Commun. 2018, 54, 11244–11259. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Sood, A.K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Karousis, N.; Suarez-Martinez, I.; Ewels, C.P.; Tagmatarchis, N. Structure, Properties, Functionalization, and Applications of Carbon Nanohorns. Chem. Rev. 2016, 116, 4850–4883. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Badea, I. Nanodiamonds as novel nanomaterials for biomedical applications: Drug delivery and imaging systems. Int. J. Nanomed. 2013, 8, 203–220. [Google Scholar] [CrossRef]
- Liu, K.K.; Cheng, C.L.; Chang, C.C.; Chao, J.I. Biocompatible and detectable carboxylated nanodiamond on human cell. Nanotechnology 2007, 18, 325102. [Google Scholar] [CrossRef]
- Bakry, R.; Vallant, R.M.; Najam-Ul-Haq, M.; Rainer, M.; Szabo, Z.; Huck, C.W.; Bonn, G.K. Medicinal applications of fullerenes. Int. J. Nanomed. 2007, 2, 639–649. [Google Scholar]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A.; Kostarelos, K.; Partidos, C.D.; Prato, M. Biomedical applications of functionalised carbon nanotubes. Chem. Commun. 2005, 5, 571–577. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Ros, T.D.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926. [Google Scholar] [CrossRef]
- Byun, J. Emerging frontiers of graphene in biomedicine. J. Microbiol. Biotechnol. 2015, 25, 145–151. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Liu, Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Chechetka, S.A.; Zhang, M.; Yudasaka, M.; Miyako, E. Physicochemically functionalized carbon nanohorns for multi-dimensional cancer elimination. Carbon 2016, 97, 45–53. [Google Scholar] [CrossRef]
- Cirillo, G.; Peitzsch, C.; Vittorio, O.; Curcio, M.; Farfalla, A.; Voli, F.; Dubrovska, A.; Iemma, F.; Kavallaris, M.; Hampel, S. When polymers meet carbon nanostructures: Expanding horizons in cancer therapy. Future Med. Chem. 2019, 11, 2205–2231. [Google Scholar] [CrossRef] [PubMed]
- Curcio, M.; Farfalla, A.; Saletta, F.; Valli, E.; Pantuso, E.; Nicoletta, F.P.; Iemma, F.; Vittorio, O.; Cirillo, G. Functionalized carbon nanostructures versus drug resistance: Promising scenarios in cancer treatment. Molecules 2020, 25, 2102. [Google Scholar] [CrossRef] [PubMed]
- Azami, T.; Kasuya, D.; Yuge, R.; Yudasaka, M.; Iijima, S.; Yoshitake, T.; Kubo, Y. Large-scale production of single-wall carbon nanohorns with high purity. J. Phys. Chem. C 2008, 112, 1330–1334. [Google Scholar] [CrossRef]
- Zhu, S.; Xu, G. Single-walled carbon nanohorns and their applications. Nanoscale 2010, 2, 2538–2549. [Google Scholar] [CrossRef]
- Chen, D.; Dougherty, C.A.; Zhu, K.; Hong, H. Theranostic applications of carbon nanomaterials in cancer: Focus on imaging and cargo delivery. J. Control. Release 2015, 210, 230–245. [Google Scholar] [CrossRef]
- Ajima, K.; Yudasaka, M.; Murakami, T.; Maigné, A.; Shiba, K.; Iijima, S. Carbon nanohorns as anticancer drug carriers. Mol. Pharm. 2005, 2, 475–480. [Google Scholar] [CrossRef]
- Guerra, J.; Herrero, M.A.; Vázquez, E. Carbon nanohorns as alternative gene delivery vectors. RSC Adv. 2014, 4, 27315–27321. [Google Scholar] [CrossRef]
- Almeida, E.R.; De Souza, L.A.; De Almeida, W.B.; Dos Santos, H.F. Molecular dynamics of carbon nanohorns and their complexes with cisplatin in aqueous solution. J. Mol. Graph. Model. 2019, 89, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, Q.; Shu, C.; Ma, X.; Li, R.; Shen, H.; Zhong, W. Targeted killing of cancer cells in vivo and in vitro with IGF-IR antibody-directed carbon nanohorns based drug delivery. Int. J. Pharm. 2015, 478, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Aryee, E.; Dalai, A.K.; Adjaye, J. Maximization of carbon nanohorns production via the Arc discharge method for hydrotreating application. J. Nanosci. Nanotechnol. 2017, 17, 4784–4791. [Google Scholar] [CrossRef]
- Albert, K.; Hsu, H.Y. Carbon-based materials for photo-triggered theranostic applications. Molecules 2016, 21, 1585. [Google Scholar] [CrossRef]
- Kagkoura, A.; Tagmatarchis, N. Carbon nanohorn-based electrocatalysts for energy conversion. Nanomaterials 2020, 10, 1407. [Google Scholar] [CrossRef]
- Zhang, M.; Yudasaka, M.; Ajima, K.; Miyawaki, J.; Iijima, S. Light-assisted oxidation of single-wall carbon nanohorns for abundant creation of oxygenated groups that enable Chemical modifications with proteins to enhance biocompatibility. ACS Nano 2007, 1, 265–272. [Google Scholar] [CrossRef]
- Aryee, E.; Dalai, A.K.; Adjaye, J. Functionalization and characterization of carbon nanohorns (CNHs) for hydrotreating of gas oils. Top. Catal. 2014, 57, 796–805. [Google Scholar] [CrossRef]
- Pagona, G.; Tagmatarchis, N.; Fan, J.; Yudasaka, M.; Iijima, S. Cone-end functionalization of carbon nanohorns. Chem. Mater. 2006, 18, 3918–3920. [Google Scholar] [CrossRef]
- Sahu, S.R.; Rikka, V.R.; Jagannatham, M.; Haridoss, P.; Chatterjee, A.; Gopalan, R.; Prakash, R. Synthesis of graphene sheets from single walled carbon nanohorns: Novel conversion from cone to sheet morphology. Mater. Res. Express 2017, 4, 035008. [Google Scholar] [CrossRef]
- Yoshida, S.; Sano, M. Microwave-assisted chemical modification of carbon nanohorns: Oxidation and Pt deposition. Chem. Phys. Lett. 2006, 433, 97–100. [Google Scholar] [CrossRef]
- Almeida, E.R.; De Souza, L.A.; De Almeida, W.B.; Dos Santos, H.F. Chemically Modified Carbon Nanohorns as Nanovectors of the Cisplatin Drug: A Molecular Dynamics Study. J. Chem. Inf. Model. 2020, 60, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Agresti, F.; Barison, S.; Famengo, A.; Pagura, C.; Fedele, L.; Rossi, S.; Bobbo, S.; Rancan, M.; Fabrizio, M. Surface oxidation of single wall carbon nanohorns for the production of surfactant free water-based colloids. J. Colloid Interface Sci. 2018, 514, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Muñiz, J.; Sansores, E.; Olea, A.; Valenzuela, E. The role of aromaticity on the building of nanohybrid materials functionalized with metalated (Au(III), Ag(III), Cu(III)) extended porphyrins and single-walled carbon nanohorns: A theoretical study. Int. J. Quantum Chem. 2013, 113, 1034–1046. [Google Scholar] [CrossRef]
- Aoyagi, M.; Yudasaka, M.; Minamikawa, H.; Asakawa, M.; Masuda, M.; Shimizu, T.; Iijima, S. Quantitative analyses of PEGylated phospholipids adsorbed on single walled carbon nanohorns by high resolution magic angle spinning 1H NMR. Carbon 2016, 101, 213–217. [Google Scholar] [CrossRef]
- Utsumi, S.; Urita, K.; Kanoh, H.; Yudasaka, M.; Suenaga, K.; Iijima, S.; Kaneko, K. Preparing a magnetically responsive single-wall carbon nanohorn colloid by anchoring magnetite nanoparticles. J. Phys. Chem. B 2006, 110, 7165–7170. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Lei, J.; Ding, L.; Ju, H. Sandwich nanohybrid of single-walled carbon nanohorns-TiO2-porphyrin for electrocatalysis and amperometric biosensing towards chloramphenicol. Chem. Commun. 2009, 28, 4227–4229. [Google Scholar] [CrossRef]
- Itoh, T.; Danjo, H.; Sasaki, W.; Urita, K.; Bekyarova, E.; Arai, M.; Imamoto, T.; Yudasaka, M.; Iijima, S.; Kanoh, H.; et al. Catalytic activities of Pd-tailored single wall carbon nanohorns. Carbon 2008, 46, 172–175. [Google Scholar] [CrossRef]
- Liu, Y.; Brown, C.M.; Neumann, D.A.; Geohegan, D.B.; Puretzky, A.A.; Rouleau, C.M.; Hu, H.; Styers-Barnett, D.; Krasnov, P.O.; Yakobson, B.I. Metal-assisted hydrogen storage on Pt-decorated single-walled carbon nanohorns. Carbon 2012, 50, 4953–4964. [Google Scholar] [CrossRef]
- Mountrichas, G.; Pispas, S.; Ichihasi, T.; Yudasaka, M.; Iijima, S.; Tagmatarchis, N. Polymer covalent functionalization of carbon nanohorns using bulk free radical polymerization. Chem. A Eur. J. 2010, 16, 5927–5933. [Google Scholar] [CrossRef]
- Ajima, K.; Yudasaka, M.; Suenaga, K.; Kasuya, D.; Azami, T.; Iijima, S. Material Storage Mechanism in Porous Nanocarbon. Adv. Mater. 2004, 16, 397–401. [Google Scholar] [CrossRef]
- Yuge, R.; Ichihashi, T.; Shimakawa, Y.; Kubo, Y.; Yudasaka, M.; Iijima, S. Preferential deposition of Pt nanoparticles inside single-walled carbon nanohorns. Adv. Mater. 2004, 16, 1420–1423. [Google Scholar] [CrossRef]
- Miyawaki, J.; Matsumura, S.; Yuge, R.; Murakami, T.; Sato, S.; Tomida, A.; Tsuruo, T.; Ichihashi, T.; Fujinami, T.; Irie, H.; et al. Biodistribution and ultrastructural localization of single-walled carbon nanohorns determined in vivo with embedded Gd2O3 labels. ACS Nano 2009, 3, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, J.; Yudasaka, M.; Azami, T.; Kubo, Y.; Iijima, S. Toxicity of single-walled carbon nanohorns. ACS Nano 2008, 2, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Lanceta, A.; Medrano-Bosch, M.; Melgar-Lesmes, P. Single-walled carbon nanohorns as promising nanotube-derived delivery systems to treat cancer. Pharmaceutics 2020, 12, 850. [Google Scholar] [CrossRef]
- Hifni, B.; Khan, M.; Devereux, S.J.; Byrne, M.H.; Quinn, S.J.; Simpson, J.C. Investigation of the Cellular Destination of Fluorescently Labeled Carbon Nanohorns in Cultured Cells. ACS Appl. Bio Mater. 2020, 3, 6790–6801. [Google Scholar] [CrossRef]
- Schramm, F.; Lange, M.; Hoppmann, P.; Heutelbeck, A. Cytotoxicity of carbon nanohorns in different human cells of the respiratory system. J. Toxicol. Environ. Health Part. A 2016, 79, 1085–1093. [Google Scholar] [CrossRef]
- Lynch, R.M.; Voy, B.H.; Glass, D.F.; Mahurin, S.M.; Zhao, B.; Hu, H.; Saxton, A.M.; Donnell, R.L.; Cheng, M.D. Assessing the pulmonary toxicity of single-walled carbon nanohorns. Nanotoxicology 2007, 1, 157–166. [Google Scholar] [CrossRef]
- Tahara, Y.; Miyawaki, J.; Zhang, M.; Yang, M.; Waga, I.; Iijima, S.; Irie, H.; Yudasaka, M. Histological assessments for toxicity and functionalization-dependent biodistribution of carbon nanohorns. Nanotechnology 2011, 22, 265106. [Google Scholar] [CrossRef]
- He, B.; Shi, Y.; Liang, Y.; Yang, A.; Fan, Z.; Yuan, L.; Zou, X.; Chang, X.; Zhang, H.; Wang, X.; et al. Single-walled carbon-nanohorns improve biocompatibility over nanotubes by triggering less protein-initiated pyroptosis and apoptosis in macrophages. Nat. Commun. 2018, 9, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Yamaguchi, T.; Iijima, S.; Yudasaka, M. Size-dependent biodistribution of carbon nanohorns in vivo. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 657–664. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Zhao, M.; Shi, Z.; Chen, X.; He, X.; Han, N.; Xu, R. Single-wall carbon nanohorns (SWNHs) inhibited proliferation of human glioma cells and promoted its apoptosis. J. Nanoparticle Res. 2013, 15, 1861. [Google Scholar] [CrossRef]
- Nowacki, M.; Wisniewski, M.; Werengowska-Ciecwierz, K.; Roszek, K.; Czarnecka, J.; Lakomska, I.; Kloskowski, T.; Tyloch, D.; Debski, R.; Pietkun, K.; et al. Nanovehicles as a novel target strategy for hyperthermic intraperitoneal chemotherapy: A multidisciplinary study of peritoneal carcinomatosis. Oncotarget 2015, 6, 22776–22798. [Google Scholar] [CrossRef] [PubMed]
- Werengowska-Ciećwierz, K.; Wiśniewski, M.; Terzyk, A.P.; Gurtowska, N.; Olkowska, J.; Kloskowski, T.; Drewa, T.A.; Kiełkowska, U.; Druzyński, S. Nanotube-mediated efficiency of cisplatin anticancer therapy. Carbon 2014, 70, 46–58. [Google Scholar] [CrossRef]
- Sarkar, S.; Gurjarpadhye, A.A.; Rylander, C.G.; Rylander, M.N. Optical properties of breast tumor phantoms containing carbon nanotubes and nanohorns. J. Biomed. Opt. 2011, 16, 051304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitney, J.; Dewitt, M.; Whited, B.M.; Carswell, W.; Simon, A.; Rylander, C.G.; Rylander, M.N. 3D viability imaging of tumor phantoms treated with single-walled carbon nanohorns and photothermal therapy. Nanotechnology 2013, 24, 275102. [Google Scholar] [CrossRef] [Green Version]
- Whitney, J.R.; Sarkar, S.; Zhang, J.; Do, T.; Young, T.; Manson, M.K.; Campbell, T.A.; Puretzky, A.A.; Rouleau, C.M.; More, K.L.; et al. Single walled carbon nanohorns as photothermal cancer agents. Lasers Surg. Med. 2011, 43, 43–51. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.; Jiang, F.; Liu, Z.; Shu, C.; Wan, L.J. In vitro and in vivo photothermally enhanced chemotherapy by single-walled carbon nanohorns as a drug delivery system. J. Mater. Chem. B 2014, 2, 4726–4732. [Google Scholar] [CrossRef]
- Yang, J.; Su, H.; Sun, W.; Cai, J.; Liu, S.; Chai, Y.; Zhang, C. Dual chemodrug-loaded single-walled carbon nanohorns for multimodal imaging-guided chemo-photothermal therapy of tumors and lung metastases. Theranostics 2018, 8, 1966–1984. [Google Scholar] [CrossRef]
- Gao, C.; Dong, P.; Lin, Z.; Guo, X.; Jiang, B.P.; Ji, S.; Liang, H.; Shen, X.C. Near-Infrared Light Responsive Imaging-Guided Photothermal and Photodynamic Synergistic Therapy Nanoplatform Based on Carbon Nanohorns for Efficient Cancer Treatment. Chem. A Eur. J. 2018, 24, 12827–12837. [Google Scholar] [CrossRef]
- Gao, C.; Jian, J.; Lin, Z.; Yu, Y.X.; Jiang, B.P.; Chen, H.; Shen, X.C. Hypericin-Loaded Carbon Nanohorn Hybrid for Combined Photodynamic and Photothermal Therapy in Vivo. Langmuir 2019, 35, 8228–8237. [Google Scholar] [CrossRef]
- Lin, Z.; Jiang, B.P.; Liang, J.; Wen, C.; Shen, X.C. Phycocyanin functionalized single-walled carbon nanohorns hybrid for near-infrared light-mediated cancer phototheranostics. Carbon 2019, 143, 814–827. [Google Scholar] [CrossRef]
- Jiang, B.P.; Hu, L.F.; Shen, X.C.; Ji, S.C.; Shi, Z.; Liu, C.J.; Zhang, L.; Liang, H. One-step preparation of a water-soluble carbon nanohorn/phthalocyanine hybrid for dual-modality photothermal and photodynamic therapy. ACS Appl. Mater. Interfaces 2014, 6, 18008–18017. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, R.; Zhang, F.; Yin, Y.; Mei, L.; Song, F.; Tao, M.; Yue, W.; Zhong, W. Overcoming multidrug resistance by a combination of chemotherapy and photothermal therapy mediated by carbon nanohorns. J. Mater. Chem. B 2016, 4, 6043–6051. [Google Scholar] [CrossRef] [PubMed]
- Gurova, O.A.; Omelyanchuk, L.V.; Dubatolova, T.D.; Antokhin, E.I.; Eliseev, V.S.; Yushina, I.V.; Okotrub, A.V. Synthesis and modification of carbon nanohorns structure for hyperthermic application. J. Struct. Chem. 2017, 58, 1205–1212. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, N.; Shu, C.; Li, R.; Ma, X.; Li, X.; Wang, R.; Zhong, W. Docetaxel-loaded single-wall carbon nanohorns using anti-VEGF antibody as a targeting agent: Characterization, in vitro and in vivo antitumor activity. J. Nanoparticle Res. 2015, 17, 207. [Google Scholar] [CrossRef]
- Ma, X.; Shu, C.; Guo, J.; Pang, L.; Su, L.; Fu, D.; Zhong, W. Targeted cancer therapy based on single-wall carbon nanohorns with doxorubicin in vitro and in vivo. J. Nanoparticle Res. 2014, 16, 2497. [Google Scholar] [CrossRef]
- Matsumura, S.; Ajima, K.; Yudasaka, M.; Iijima, S.; Shiba, K. Dispersion of cisplatin-loaded carbon nanohorns with a conjugate comprised of an artificial peptide aptamer and polyethylene glycol. Mol. Pharm. 2007, 4, 723–729. [Google Scholar] [CrossRef]
- Shanmugam, V.; Selvakumar, S.; Yeh, C.S. Near-infrared light-responsive nanomaterials in cancer therapeutics. Chem. Soc. Rev. 2014, 43, 6254–6287. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, K.A.; Inglefield, D.L., Jr.; Zhang, J.; Dorn, H.C.; Long, T.E.; Rylander, C.G.; Rylander, M.N. Single-walled carbon nanohorns decorated with semiconductor quantum dots to evaluate intracellular transport. J. Nanoparticle Res. 2014, 16, 2078. [Google Scholar] [CrossRef]
- DeWitt, M.R.; Pekkanen, A.M.; Robertson, J.; Rylander, C.G.; Rylander, M.N. Influence of hyperthermia on efficacy and uptake of carbon nanohorn-cisplatin conjugates. J. Biomech. Eng. 2014, 136, 021003. [Google Scholar] [CrossRef] [Green Version]
- Isaac, K.M.; Sabaraya, I.V.; Ghousifam, N.; Das, D.; Pekkanen, A.M.; Romanovicz, D.K.; Long, T.E.; Saleh, N.B.; Rylander, M.N. Functionalization of single-walled carbon nanohorns for simultaneous fluorescence imaging and cisplatin delivery in vitro. Carbon 2018, 138, 309–318. [Google Scholar] [CrossRef]
- Chechetka, S.A.; Pichon, B.; Zhang, M.; Yudasaka, M.; Bégin-Colin, S.; Bianco, A.; Miyako, E. Multifunctional carbon nanohorn complexes for cancer treatment. Chem. Asian J. 2015, 10, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Chechetka, S.A.; Yuba, E.; Kono, K.; Yudasaka, M.; Bianco, A.; Miyako, E. Magnetically and near-infrared light-powered supramolecular nanotransporters for the remote control of enzymatic reactions. Angew. Chem. Int. Ed. 2016, 55, 6476–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Murakami, T.; Ajima, K.; Tsuchida, K.; Sandanayaka, A.S.D.; Ito, O.; Iijima, S.; Yudasaka, M. Fabrication of ZnPc/protein nanohorns for double photodynamic and hyperthermic cancer phototherapy. Proc. Natl. Acad. Sci. USA 2008, 105, 14773–14778. [Google Scholar] [CrossRef] [Green Version]
- Lucío, M.I.; Opri, R.; Pinto, M.; Scarsi, A.; Fierro, J.L.G.; Meneghetti, M.; Fracasso, G.; Prato, M.; Vázquez, E.; Herrero, M.A. Targeted killing of prostate cancer cells using antibody-drug conjugated carbon nanohorns. J. Mater. Chem. B 2017, 5, 8821–8832. [Google Scholar] [CrossRef]
- Pérez-Martínez, F.C.; Carrión, B.; Lucío, M.I.; Rubio, N.; Herrero, M.A.; Vázquez, E.; Ceña, V. Enhanced docetaxel-mediated cytotoxicity in human prostate cancer cells through knockdown of cofilin-1 by carbon nanohorn delivered siRNA. Biomaterials 2012, 33, 8152–8159. [Google Scholar] [CrossRef]
- Guerra, J.; Herrero, M.A.; Carrión, B.; Pérez-Martínez, F.C.; Lucío, M.; Rubio, N.; Meneghetti, M.; Prato, M.; Ceña, V.; Vázquez, E. Carbon nanohorns functionalized with polyamidoamine dendrimers as efficient biocarrier materials for gene therapy. Carbon 2012, 50, 2832–2844. [Google Scholar] [CrossRef]
- Cirillo, G.; Caruso, T.; Hampel, S.; Haase, D.; Puoci, F.; Ritschel, M.; Leonhardt, A.; Curcio, M.; Iemma, F.; Khavrus, V.; et al. Novel carbon nanotube composites by grafting reaction with water-compatible redox initiator system. Colloid Polym. Sci. 2013, 291, 699–708. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Chen, Y.; Chen, Z.; Chen, C.; Li, Y.; Cui, Z.; Zhang, D. Grafting of graphene oxide with stimuli-responsive polymers by using ATRP for drug release. J. Nanoparticle Res. 2012, 14, 1132. [Google Scholar] [CrossRef]
- Hattori, Y.; Kanoh, H.; Okino, F.; Touhara, H.; Kasuya, D.; Yudasaka, M.; Iijima, S.; Kaneko, K. Direct thermal fluorination of single wall carbon nanohorns. J. Phys. Chem. B 2004, 108, 9614–9618. [Google Scholar] [CrossRef]
- Isobe, H.; Tanaka, T.; Maeda, R.; Noiri, E.; Solin, N.; Yudasaka, M.; Iijima, S.; Nakamura, E. Preparation, purification, characterization, and cytotoxicity assessment of water-soluble, transition-metal-free carbon nanotube aggregates. Angew. Chem. Int. Ed. 2006, 45, 6676–6680. [Google Scholar] [CrossRef] [PubMed]
- Lacotte, S.; García, A.; Décossas, M.; Al-Jamal, W.T.; Li, S.; Kostarelos, K.; Muller, S.; Prato, M.; Dumortier, H.; Bianco, A. Interfacing functionalized carbon nanohorns with primary phagocytic cells. Adv. Mater. 2008, 20, 2421–2426. [Google Scholar] [CrossRef]
- Pagona, G.; Karousis, N.; Tagmatarchis, N. Aryl diazonium functionalization of carbon nanohorns. Carbon 2008, 46, 604–610. [Google Scholar] [CrossRef]
- Tagmatarchis, N.; Maigné, A.; Yudasaka, M.; Iijima, S. Functionalization of carbon nanohorns with azomethine ylides: Towards solubility enhancement and electron-transfer processes. Small 2006, 2, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, M.; Gómez-Escalonilla, M.J.; Fierro, J.L.G.; Yudasaka, M.; Iijima, S.; Vartanian, M.; Iehl, J.; Nierengarten, J.F.; Langa, F. A soluble hybrid material combining carbon nanohorns and C60. Chem. Commun. 2011, 47, 12771–12773. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.; Campidelli, S.; Brunetti, F.G.; Meneghetti, M.; Prato, M. Functionalisation of carbon nanohorns. Chem. Commun. 2006, 20, 2129–2131. [Google Scholar] [CrossRef]
- Herrero, M.A.; Prato, M. Recent advances in the covalent functionalization of carbon nanotubes. Mol. Cryst. Liq. Cryst. 2008, 483, 21–32. [Google Scholar] [CrossRef]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of Graphene via 1,3-Dipolar Cycloaddition. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [Green Version]
- Battigelli, A.; Menard-Moyon, C.; Da Ros, T.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef]






| Carrier Features | Biological Features | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CNH | Derivatizating Agent | Targeting Element | Bioactive Agent (DL%) | Cancer Model | Health Model | Performance | ||||
| Tissue | In Vitro | In Vivo | In Vitro | In Vivo | ||||||
| CNH | - - - | - - - | CDDP a (33/50) | - - - | - - - | - - - | - - - | - - - | Slow release | [63] |
| SWCNH | PS | - - - | - - - | Brain | U87 | - - - | - - - | - - - | Apoptosis | [93] |
| U251 | ||||||||||
| U373 | ||||||||||
| SWCNH | PF127 | - - - | - - - | - - - | - - - | - - - | - - - | - - - | Photothermal | [96] |
| SWCNH | PF127 | - - - | - - - | Breast | MDA-MB-231 | - - - | - - - | - - - | Photothermal | [97] |
| SWCNH | PF127 | - - - | - - - | Kidney | RENCA | - - - | - - - | - - - | Photothermal | [98] |
| SWCNH | DCA-HPCS | - - - | DOX b | Breast | 4T1 | 4T1 | - - - | - - - | Photothermal synergism | [99] |
| SWCNH | C18PMH/mPEG-PLA | pH | CDDP b (66) DOX b (44) | Breast | 4T1 | 4T1 | - - - | - - - | Photothermal synergism | [100] |
| Lung | - - - | |||||||||
| SWCNH | - - - | - - - | ICG b (37) | Breast | 4T1 | 4T1 | - - - | - - - | Photodynamic | [101] |
| SWCNH | - - - | - - - | HYP b (52) | Breast | 4T1 | 4T1 | - - - | - - - | Photodynamic | [102] |
| SWCNH | - - - | - - - | PC b (33) | Breast | 4T1 | 4T1 | - - - | Balb/c mice | Photodynamic | [103] |
| MDA-MB-231 | ||||||||||
| SWCNH | - - - | - - - | TSCuPc b (36) | Cervix | HeLa | - - - | - - - | - - - | Photodynamic | [104] |
| oxCNH | DISPE-PEG | P-gp Ab | ETO b (40) | Lung | A549 | - - - | - - - | - - - | Photothermal synergism MDR reversal | [105] |
| A549R | A549R | |||||||||
| 1 oxSWCNH | - - - | - - - | - - - | - - - | - - - | - - - | - - - | - - - | Photothermal | [106] |
| 2 oxSWCNH | - - - | - - - | CDDP a (20) | Lung | NCI-H460 | - - - | - - - | - - - | Synergism | [61] |
| oxSWCNH | DISPE-PEG | VEGF mAb | DTX b (31) | Breast | MCF7 | - - - | - - - | - - - | Synergism | [107] |
| Liver | - - - | H22 | ||||||||
| oxSWCNH | SA | pH VEGF mAb | DOX b (50) | Breast | MCF7 | - - - | - - - | - - - | Synergism | [108] |
| Kidney | - - - | - - - | HEK293 | |||||||
| Liver | - - - | H22 | - - - | |||||||
| oxSWCNH | DISPE-PEG | IGF-IR mAb | VCR b (38) | Breast | MCF7 | - - - | HUVEC | - - - | Synergism | [64] |
| Liver | - - - | H22 | ||||||||
| 2 oxSWCNH | PEG–NHBP | - - - | CDDP a (22) | Lung | NCI-H460 | - - - | - - - | - - - | Synergism | [109] |
| Carrier Features | Biological Features | Ref. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CNH | Derivatization | Targeting | Bioactive Agent (DL%) | Cancer Model | Health Model | Performance | |||||
| Derivatizing Agent | Synthesis | Tissue | In Vitro | In Vivo | In Vitro | In Vivo | |||||
| 1 oxSWCNH | AET CdSe/ZnS QDs | Condensation (EDC)/ Coordination | - - - | - - - | Breast | MDA-MB-231 | - - - | - - - | - - - | High uptake | [111] |
| Brain | U-87 | ||||||||||
| Bladder | AY-27 | ||||||||||
| 1 oxSWCNH | CDDP | Condensation (EDC) | - - - | - - - | Bladder | AY-27 | - - - | - - - | - - - | High uptake Photothermal | [112] |
| - - - | - - - | - - - | CDDP a | ||||||||
| AET CdSe/ZnS QDs | Condensation (EDC)/ Coordination | - - - | - - - | ||||||||
| 1 oxSWCNH | AET CdSe/ZnS QDs | Condensation (EDC)/ Coordination | - - - | CDDP a (19) | Bladder | AY-27 | - - - | - - - | - - - | Synergism Fluorescence Imaging | [113] |
| 2 oxCNH | PEI | Condensation (EDC) | Magnetic | MAG | Cervix | HeLa | - - - | - - - | - - - | Photothermal | [114] |
| 2 oxCNH | PEI | Condensation (EDC) | Magnetic FA | MAG | Epidermis | KB | - - - | FHs173We | - - - | Photothermal | [55] |
| 2 oxCNH | Liposome-AVI-BIOT-PEI | Condensation (EDC) | Magnetic | MAG | Cervix | HeLa | - - - | GP8 | Mice | Photothermal | [115] |
| SV40 | |||||||||||
| 3 oxSWCNH | BSA/ZnPc | Condensation (EDC)/Wrapping | --- | --- | Modified Fibroblast | 5RP7 | 5RP7 | - - - | - - - | Photothermal | [116] |
| CNH | PSMA mAb | Cycloaddition | PSMA mAb | CDDP b (1.3) | Prostate | PC-3 | - - - | - - - | - - - | Synergism | [117] |
| CNH | PAMAM | Cycloaddition | - - - | siRNA b DTX c | Prostate | LNCaP | - - - | - - - | - - - | Synergism | [118] |
| CNH | PAMAM/ AuNPs | Cycloaddition/Coordination | - - - | siRNA b | Prostate | PC3 | - - - | - - - | - - - | Synergism | [119] |
| CNH | Approach | Ref. | Total Studies * | Derivatizing Agent | Reaction | Targeting | Drug | Cancer Model | Efficacy | Side-Toxicity | Performance |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % Studies # | % Success # | ||||||||||
| CNH | Non covalent | [63] [93] [96] [97] [98] [99] [100] [101] [102] [103] [104] | 12 | None (42) PF (25) Other (33) | None (33) Wrapping (77) | None (83) Stimuli (17) | None (35) CDDP (25) DOX (8) ICG (8) HYP (8) PC (8) TsCuPc (8) | None (18) Breast (50) Brain (8) Kidney (8) Lung (8) Cervix (8) | Vitro (75) Vivo (50) | 0 | Release (8) Apoptosis (8) Synergism (25) Phototherapy (75) |
| Covalent | [117] [118] [119] | 3 | PAMAM (67) Ab (33) | Cycloaddition (100) | Ab (33) | CDDP (33) SiRNA (66) DTX (33) | Prostate (100) | Vitro (100) | Synergism (100) | ||
| oxCNH | Non covalent | [61] [64] [105,106] [107] [108] [109] | 9 | None (22) dPEG (67) SA (11) | None (22) Wrapping (78) | None (33) Ab (66) pH (11) | None (11) CDDP (22) DTX (22) DOX (11) VCR (22) ETO (11) | None (11) Lung (33) Breast (33) Liver (22) | Vitro (67) Vivo (33) | 22 | Synergism (78) Phototherapy (22) MDR reversal (11) |
| oxCNH | Covalent | [55] [111] [112] [113] [114] [115] [116] | 11 | None (9) QDs (45) PEI (27) BSA (9) CDDP (9) | Condensation (100) | Magnetic (27) FA (9) | None (85) CDDP (27) MAG (27) | Bladder (45) Cervix (18) Breast (9) Brain (9) Epidermis (9) Fibroblast (9) | Vitro (100) Vivo (9) | 18 | Synergism (36) Photherapy (63) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curcio, M.; Cirillo, G.; Saletta, F.; Michniewicz, F.; Nicoletta, F.P.; Vittorio, O.; Hampel, S.; Iemma, F. Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy. C 2021, 7, 3. https://doi.org/10.3390/c7010003
Curcio M, Cirillo G, Saletta F, Michniewicz F, Nicoletta FP, Vittorio O, Hampel S, Iemma F. Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy. C. 2021; 7(1):3. https://doi.org/10.3390/c7010003
Chicago/Turabian StyleCurcio, Manuela, Giuseppe Cirillo, Federica Saletta, Filip Michniewicz, Fiore Pasquale Nicoletta, Orazio Vittorio, Silke Hampel, and Francesca Iemma. 2021. "Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy" C 7, no. 1: 3. https://doi.org/10.3390/c7010003
APA StyleCurcio, M., Cirillo, G., Saletta, F., Michniewicz, F., Nicoletta, F. P., Vittorio, O., Hampel, S., & Iemma, F. (2021). Carbon Nanohorns as Effective Nanotherapeutics in Cancer Therapy. C, 7(1), 3. https://doi.org/10.3390/c7010003