Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer
Abstract
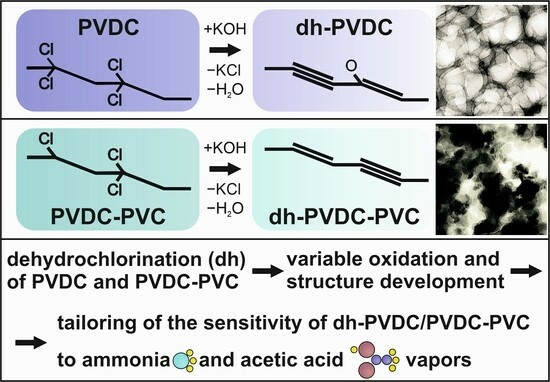
:1. Introduction
2. Experimental Section
2.1. Formation of Polyyne–Polyenes and Sp-Based Sensors
2.2. Characterization of the Samples’ Structure
2.3. Sensing Response Measurements
3. Results
3.1. TEM
3.2. Raman Spectroscopy
3.3. FTIR
3.4. Sensing Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application. Chemosensors 2021, 9, 198. [Google Scholar] [CrossRef]
- Musayeva, N.; Khalilova, H.; Izzatov, B.; Trevisi, G.; Ahmadova, S.; Alizada, M. Highly Selective Detection of Hydrogen Sulfide by Simple Cu-CNTs Nanocomposites. C 2023, 9, 25. [Google Scholar] [CrossRef]
- Zavidovskiy, I.A.; Streletskiy, O.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Savchenko, N.F.; Tatarintsev, A.A.; Pavlikov, A.V. Highly Selective Polyene-Polyyne Resistive Gas Sensors: Response Tuning by Low-Energy Ion Irradiation. J. Compos. Sci. 2023, 7, 156. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Bandosz, T.J. Defectous UiO-66 MOF Nanocomposites as Reactive Media of Superior Protection against Toxic Vapors. ACS Appl. Mater. Interfaces 2020, 12, 14678–14689. [Google Scholar] [CrossRef]
- Shybanov, D.E.; Filkina, M.E.; Kukushkin, M.E.; Grishin, Y.K.; Roznyatovsky, V.A.; Zyk, N.V.; Beloglazkina, E.K. Diffusion Mixing with a Volatile Tertiary Amine as a Very Efficient Technique for 1,3-Dipolar Cycloaddition Reactions Proceeding via Dehydrohalogenation of Stable Precursors of Reactive Dipoles. New J. Chem. 2022, 46, 18575–18586. [Google Scholar] [CrossRef]
- Qu, P.; Liu, X.; Wang, S.; Xiao, C.; Liu, S. Moderate Dehydrofluorinated PVDF with High Energy Density. Mater. Lett. 2018, 221, 275–278. [Google Scholar] [CrossRef]
- González, S.; Porras, M.; Jimbo, A.; Zambrano, C.H. Dehydrochlorination of PCDDs on SWCN-Supported Ni10 and Ni13 Clusters, a DFT Study. Molecules 2022, 27, 5074. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Khaidarov, A.A.; Pavlikov, A.V.; Minnebaev, K.F. The Field-Effect Transistor Based on a Polyyne–Polyene Structure Obtained via PVDC Dehydrochlorination. J. Compos. Sci. 2023, 7, 264. [Google Scholar] [CrossRef]
- Pan, B.; Xiao, J.; Li, J.; Liu, P.; Wang, C.; Yang, G. Carbyne with Finite Length: The One-Dimensional Sp Carbon. Sci. Adv. 2015, 1, e1500857. [Google Scholar] [CrossRef]
- Eaton, A.L.; Fielder, M.; Nair, A.K. Mechanical and Thermal Properties of Carbon-Based Low-Dimensional Materials. MRS Bull. 2022, 47, 1001–1010. [Google Scholar] [CrossRef]
- Zanolli, Z.; Onida, G.; Charlier, J.-C. Quantum Spin Transport in Carbon Chains. ACS Nano 2010, 4, 5174–5180. [Google Scholar] [CrossRef]
- La Torre, A.; Botello-Mendez, A.; Baaziz, W.; Charlier, J.-C.; Banhart, F. Strain-Induced Metal–Semiconductor Transition Observed in Atomic Carbon Chains. Nat. Commun. 2015, 6, 6636. [Google Scholar] [CrossRef]
- Wang, M.; Lin, S. Ballistic Thermal Transport in Carbyne and Cumulene with Micron-Scale Spectral Acoustic Phonon Mean Free Path. Sci. Rep. 2015, 5, 18122. [Google Scholar] [CrossRef]
- Hukka, T.I.; Pakkanen, T.A.; D’Evelyn, M.P. Chemisorption of Fluorine, Chlorine, HF, and HCl on the Diamond (100)2x1 Surface: An Ab Initio Study. J. Phys. Chem. 1995, 99, 4710–4719. [Google Scholar] [CrossRef]
- Xu, B.; Hou, S.; Chu, M.; Cao, G.; Yang, Y. An Activation-Free Method for Preparing Microporous Carbon by the Pyrolysis of Poly(Vinylidene Fluoride). Carbon 2010, 48, 2812–2814. [Google Scholar] [CrossRef]
- Chaudhary, V.; Gautam, A.; Mishra, Y.K.; Kaushik, A. Emerging MXene–Polymer Hybrid Nanocomposites for High-Performance Ammonia Sensing and Monitoring. Nanomaterials 2021, 11, 2496. [Google Scholar] [CrossRef]
- Evsyukov, S.E. Chemical Dehydrohalogenation of Polymers. In Carbyne and Carbynoid Structures; Heimann, R.B., Evsyukov, S.E., Kavan, L., Eds.; Physics and Chemistry of Materials with Low-Dimensional Structures; Springer: Dordrecht, The Netherlands, 1999; Volume 21, pp. 55–74. ISBN 978-94-010-5993-0. [Google Scholar]
- Streletskiy, O.; Zavidovskiy, I.; Yakubovsky, D.; Doroshina, N.; Syuy, A.; Lebedinskij, Y.; Markeev, A.; Arsenin, A.; Volkov, V.; Novikov, S. Tailoring of the Distribution of SERS-Active Silver Nanoparticles by Post-Deposition Low-Energy Ion Beam Irradiation. Materials 2022, 15, 7721. [Google Scholar] [CrossRef]
- McGivern, W.S.; Derecskei-Kovacs, A.; North, S.W.; Francisco, J.S. Computationally Efficient Methodology to Calculate C−H and C−X (X = F, Cl, and Br) Bond Dissociation Energies in Haloalkanes. J. Phys. Chem. A 2000, 104, 436–442. [Google Scholar] [CrossRef]
- Cioslowski, J.; Liu, G.; Moncrieff, D. Energetics of the Homolytic C−H and C−Cl Bond Cleavages in Polychlorobenzenes: The Role of Electronic and Steric Effects. J. Phys. Chem. A 1997, 101, 957–960. [Google Scholar] [CrossRef]
- Abdu, Y.A. Raman Micro-Spectroscopy of Nanodiamonds from the Kapoeta Meteorite. Diam. Relat. Mater. 2021, 118, 108536. [Google Scholar] [CrossRef]
- Zhivulin, V.E.; Khairanov, R.K.; Zlobina, N.A.; Pesin, L.A. Modification of the IR Spectra Shape in the 2000–2300 Cm–1 Absorption Band upon the Aging of a Chemically Dehydrofluorinated Poly(Vinylidene Fluoride) Film. J. Surf. Investig. 2020, 14, 1144–1151. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Nishchak, O.Y.; Zavidovskiy, I.A.; Maslakov, K.I.; Pavlikov, A.V. Sp-Based Thin Films Synthesized by Magnetron Sputtering of Dehydrohalogenated Polyvinylidenchloride. Thin Solid Film. 2021, 739, 138993. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nishchak, O.Y.; Khaidarov, A.A.; Savchenko, N.F.; Pavlikov, A.V. Low-Threshold Field Emission Cathode Based on Heat-Treated Dehydrofluorinated Polyvinylidene Fluoride. J. Exp. Theor. Phys. 2022, 135, 844–852. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Balabanyan, V.Y.; Tsiskarashvili, A.V. Antibacterial Properties of Modified A-C and Ta-C Coatings: The Effects of the Sp2/Sp3 Ratio, Oxidation, Nitridation, and Silver Incorporation. Appl. Phys. A 2022, 128, 929. [Google Scholar] [CrossRef]
- Novikov, V.S.; Kuzmin, V.V.; Kuznetsov, S.M.; Darvin, M.E.; Lademann, J.; Sagitova, E.A.; Ustynyuk, L.Y.; Prokhorov, K.A.; Nikolaeva, G.Y. DFT Study of Raman Spectra of Polyenes and SS-Carotene: Dependence on Length of Polyene Chain and Isomer Type. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119668. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, T.; Yamazato, M.; Higa, A.; Toguchi, M. Raman Analysis of Trans -Polyacetylene Chains in Hydrogenated Amorphous Carbon Films. Jpn. J. Appl. Phys. 2007, 46, 756–760. [Google Scholar] [CrossRef]
- Brambilla, L.; Tommasini, M.; Zerbi, G.; Stradi, R. Raman Spectroscopy of Polyconjugated Molecules with Electronic and Mechanical Confinement: The Spectrum of Corallium Rubrum: Raman Spectroscopy of Polyconjugated Molecules with Electronic and Mechanical Confinement. J. Raman Spectrosc. 2012, 43, 1449–1458. [Google Scholar] [CrossRef]
- Buntov, E.A.; Zatsepin, A.F.; Guseva, M.B.; Ponosov, Y.S. 2D-Ordered Kinked Carbyne Chains: DFT Modeling and Raman Characterization. Carbon 2017, 117, 271–278. [Google Scholar] [CrossRef]
- Coleman, M.M.; Wu, M.S.; Harrison, I.R.; Painter, P.C. Vibrational Spectra and Conformation of Poly(Vinylidene Chloride). J. Macromol. Sci. Part B 1978, 15, 463–480. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef]
- Pamuła, E.; Błażewicz, M.; Paluszkiewicz, C.; Dobrzyński, P. FTIR Study of Degradation Products of Aliphatic Polyesters–Carbon Fibres Composites. J. Mol. Struct. 2001, 596, 69–75. [Google Scholar] [CrossRef]
- Burket, C.L.; Rajagopalan, R.; Marencic, A.P.; Dronvajjala, K.; Foley, H.C. Genesis of Porosity in Polyfurfuryl Alcohol Derived Nanoporous Carbon. Carbon 2006, 44, 2957–2963. [Google Scholar] [CrossRef]
- Karimov, O.K.; Teptereva, G.A.; Chetvertneva, I.A.; Movsumzade, E.M.; Karimov, E.K. The Structure of Lignosulfonates for Production of Carbon Catalyst Support. IOP Conf. Ser. Earth Environ. Sci. 2021, 839, 022086. [Google Scholar] [CrossRef]
- Venkatesh, R.; Karthi, N.; Kawin, N.; Prakash, T.; Kannan, C.R.; Karthigairajan, M.; Bobe, K. Synthesis and Adsorbent Performance of Modified Biochar with Ag/MgO Nanocomposites for Heat Storage Application. Adsorpt. Sci. Technol. 2022, 2022, e7423102. [Google Scholar] [CrossRef]
- Kaur, G.A.; Sharma, V.; Gupta, N.; Shandilya, M.; Rai, R. Structural and Optical Amendment of PVDF into CQDs through High Temperature Calcination Process. Mater. Lett. 2021, 304, 130616. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Concepción, P.; Knözinger, H. Analysis of Oxidation States of Vanadium in Vanadia–Titania Catalysts by the IR Spectra of Adsorbed NO. Top. Catal. 2000, 11, 123–130. [Google Scholar] [CrossRef]
- Neto, E.L.d.O.; Cezar, J.G.; Doro, F.G.; Carvalho, J.R.M.; Ferreira, K.Q. Synthesis, Characterization and Reactivity of Nitrosyl Ruthenium Complexes with the Non-Stereoidal Anti-Inflammatory Diflunisal. J. Chem. Environ. Biol. Eng. 2020, 4, 39. [Google Scholar] [CrossRef]
- Lucotti, A.; Tommasini, M.; Fazzi, D.; Del Zoppo, M.; Chalifoux, W.A.; Ferguson, M.J.; Zerbi, G.; Tykwinski, R.R. Evidence for Solution-State Nonlinearity of Sp-Carbon Chains Based on IR and Raman Spectroscopy: Violation of Mutual Exclusion. J. Am. Chem. Soc. 2009, 131, 4239–4244. [Google Scholar] [CrossRef]
- Leong, T.X.; Collins, B.K.; Dey Baksi, S.; Mackin, R.T.; Sribnyi, A.; Burin, A.L.; Gladysz, J.A.; Rubtsov, I.V. Tracking Energy Transfer across a Platinum Center. J. Phys. Chem. A 2022, 126, 4915–4930. [Google Scholar] [CrossRef]
- Begni, F.; Paul, G.; Lasseuguette, E.; Mangano, E.; Bisio, C.; Ferrari, M.-C.; Gatti, G. Synthetic Saponite Clays as Additives for Reducing Aging Effects in PIM1 Membranes. ACS Appl. Polym. Mater. 2020, 2, 3481–3490. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Alexander, C.M.O.; Cody, G.D. Comparison of FT-IR Spectra of Bulk and Acid Insoluble Organic Matter in Chondritic Meteorites: An Implication for Missing Carbon during Demineralization. Meteorit. Planet. Sci. 2019, 54, 1632–1641. [Google Scholar] [CrossRef]
- Siriwong, C.; Khansawai, P.; Boonchiangma, S.; Sirisinha, C.; Sae-Oui, P. The Influence of Modified Soybean Oil as Processing Aids in Tire Application. Polym. Bull. 2021, 78, 3589–3606. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, Y.; Li, Q.; Yan, W.; Zhang, X.; Ouyang, X.; Ouyang, X.; Chen, L.; Liao, B. A Study of Al2O3/MgO Composite Films Deposited by FCVA for Thin-Film Encapsulation. Materials 2023, 16, 1955. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Ling-Chung, S.K. Conducting Polymer Gas Sensors Part II: Response of Polypyrrole to Methanol Vapour. Sens. Actuators 1989, 19, 141–150. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting Polymers: A Comprehensive Review on Recent Advances in Synthesis, Properties and Applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia Gas Sensors: A Comprehensive Review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Muangrat, W.; Obata, M.; Hashimoto, Y. Enhancement Sensitivity and Selectivity of Ammonium Hydroxide Using Nitrogen-Doped Double-Walled Carbon Nanotubes. Trends Sci. 2022, 19, 2891. [Google Scholar] [CrossRef]
- Ahmadi, S.; Afzalzadeh, R. Few-Layer Graphene Doped with Boron to Enhance Ammonium Hydroxide Vapour Detection at Low Temperature. Micro Nano Lett. 2018, 13, 363–368. [Google Scholar] [CrossRef]
- Liu, K.; Yang, P.; Li, S.; Li, J.; Ding, T.; Xue, G.; Chen, Q.; Feng, G.; Zhou, J. Induced Potential in Porous Carbon Films through Water Vapor Absorption. Angew. Chem. Int. Ed. 2016, 55, 8003–8007. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, A.; Nuthalapati, S.; Nag, A.; Afsarimanesh, N.; Alahi, M.E.E.; Altinsoy, M.E. A Critical Review of the Use of Graphene-Based Gas Sensors. Chemosensors 2022, 10, 355. [Google Scholar] [CrossRef]
- Cheng, L.; Ma, S.Y.; Li, X.B.; Luo, J.; Li, W.Q.; Li, F.M.; Mao, Y.Z.; Wang, T.T.; Li, Y.F. Highly Sensitive Acetone Sensors Based on Y-Doped SnO2 Prismatic Hollow Nanofibers Synthesized by Electrospinning. Sens. Actuators B Chem. 2014, 200, 181–190. [Google Scholar] [CrossRef]
- Gong, Y.; Li, H.; Pei, W.; Fan, J.; Umar, A.; Al-Assiri, M.S.; Wang, Y.; Rooij, N.F.d.; Zhou, G. Assembly with Copper(II) Ions and D–π–A Molecules on a Graphene Surface for Ultra-Fast Acetic Acid Sensing at Room Temperature. RSC Adv. 2019, 9, 30432–30438. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Zhang, Q.Q.; Ma, S.Y.; Wan, G.X.; Li, F.M.; Xu, X.L. Microstructure Optimization and Gas Sensing Improvement of ZnO Spherical Structure through Yttrium Doping. Sens. Actuators B Chem. 2014, 195, 526–533. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Yoon, H.; Jang, J. Highly Sensitive and Selective Chemiresistive Sensors Based on Multidimensional Polypyrrole Nanotubes. Chem. Commun. 2012, 48, 10526–10528. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Shen, W.; Chen, W.; Tan, R.; Xu, L.; Song, W. PSS-PANI/PVDF Composite Based Flexible NH3 Sensors with Sub-Ppm Detection at Room Temperature. Sens. Actuators B Chem. 2021, 328, 129085. [Google Scholar] [CrossRef]
- Wu, Q.; Shen, W.; Lv, D.; Chen, W.; Song, W.; Tan, R. An Enhanced Flexible Room Temperature Ammonia Gas Sensor Based on GP-PANI/PVDF Multi-Hierarchical Nanocomposite Film. Sens. Actuators B Chem. 2021, 334, 129630. [Google Scholar] [CrossRef]
- Pagidi, S.; Pasupuleti, K.S.; Reddeppa, M.; Ahn, S.; Kim, Y.; Kim, J.-H.; Kim, M.-D.; Lee, S.H.; Jeon, M.Y. Resistive Type NO2 Gas Sensing in Polymer-Dispersed Liquid Crystals with Functionalized-Carbon Nanotubes Dopant at Room Temperature. Sens. Actuators B Chem. 2022, 370, 132482. [Google Scholar] [CrossRef]
- Zhao, Q.; Yuan, Z.; Duan, Z.; Jiang, Y.; Li, X.; Li, Z.; Tai, H. An Ingenious Strategy for Improving Humidity Sensing Properties of Multi-Walled Carbon Nanotubes via Poly-L-Lysine Modification. Sens. Actuators B Chem. 2019, 289, 182–185. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, L.; Wang, J.; Zhuang, R.; Mu, P.; Wang, J.; Yan, W. Advances in Functional Guest Materials for Resistive Gas Sensors. RSC Adv. 2022, 12, 24614–24632. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Pavlikov, A.V.; Savchenko, N.F. Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C 2023, 9, 82. https://doi.org/10.3390/c9030082
Streletskiy OA, Zavidovskiy IA, Nuriahmetov IF, Nishchak OY, Pavlikov AV, Savchenko NF. Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C. 2023; 9(3):82. https://doi.org/10.3390/c9030082
Chicago/Turabian StyleStreletskiy, Oleg A., Ilya A. Zavidovskiy, Islam F. Nuriahmetov, Olesya Y. Nishchak, Alexander V. Pavlikov, and Natalya F. Savchenko. 2023. "Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer" C 9, no. 3: 82. https://doi.org/10.3390/c9030082
APA StyleStreletskiy, O. A., Zavidovskiy, I. A., Nuriahmetov, I. F., Nishchak, O. Y., Pavlikov, A. V., & Savchenko, N. F. (2023). Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C, 9(3), 82. https://doi.org/10.3390/c9030082