Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass
Abstract
:1. Introduction
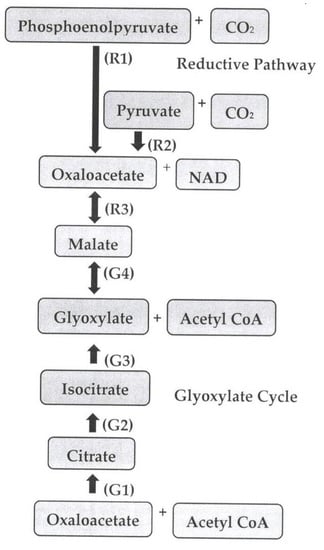
2. Pathways of Malic Acid Biosynthesis
3. Microbial Malic Acid Production from Sugars
4. Malic acid Production from Biofuel-Related Coproducts
5. Lignocellulosic Biomass-Based Malic Acid Production
6. Malic Acid Derived from poly(β-l-malic acid) (PMA) Production
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mondala, A.H. Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric and malic acids: Current and future prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Microbial malic acid production: Exploring new avenues of synthesizing a commercially-valuable chemical. J. Microb. Biochem. Technol. 2016, 8, 321. [Google Scholar] [CrossRef]
- Khan, I.; Nazir, K.; Wang, Z.-P.; Liu, G.-L.; Chi, Z.-M. Calcium malate overproduction by Penicillium viticola 152 using the medium containing corn steep liquor. Appl. Microbiol. Biotechnol. 2014, 98, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Mao, Y.; Zhang, X. Metabolic engineering of a laboratory-evolved Thermobifida fusca muC strain for malic acid production on cellulose and minimal treated lignocellulose. Biotechnol. Prog. 2016, 32, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, R.J.; Peleg, Y.; Rokem, J.S.; Pines, O.; Goldberg, I. l-Malic acid formation by immobilized Saccharomyces cerevisiae amplified for fumarase. Enzym. Microb. Technol. 1991, 13, 991–996. [Google Scholar] [CrossRef]
- Leathers, T.D.; Manitchotpisit, P. Production of poly(β-l-malic acid) (PMA) from agricultural biomass substrates by Aureobasidium pullulans. Biotechnol. Lett. 2013, 35, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhou, Y.; Yang, S.T. Production of polymalic acid and malic acid by Aureobasidium pullulans. Biotechnol. Bioeng. 2013, 110, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, G.L.; Liu, C.-G.; Chi, Z.-M. Poly(β-l-malic acid) (PMA) from Aureobasidium spp. and its current proceedings. Appl. Microbiol. Biotechnol. 2016, 100, 3841–3851. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Wang, Y.; Tu, G.; Zan, Z.; Wu, X. Adaption and transcriptome analysis of Aureobasidium pullulans in corncob hydrolysate for increased inhibitor tolerance to malic acid production. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Cao, W.; Chen, X.; Luo, J.; Yin, J.; Qiao, C.; Wan, Y. High molecular weight poly(β-l-malic acid) produced by A. pullulans with Ca2+ added repeated batch culture. Int. J. Biol. Macromol. 2016, 85, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.H.; Bashkirova, L.; Berka, R.; Chandler, T.; Doty, T.; McCall, K.; McCulloch, M.; McFarland, S.; Thompson, S.; Yaver, D.; Berry, A. Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of l-malic acid. Appl. Microbiol. Biotechnol. 2013, 97, 8903–8912. [Google Scholar] [CrossRef] [PubMed]
- Oba, T.; Kusomoto, K.; Kichise, Y.; Izumoto, E.; Nakayama, S.; Tashiro, K.; Kuhara, S.; Kitagaki, H. Variations in mitochondrial membrane potential correlate with malic acid production by natural isolates of Saccharomyces cerevisiae sake strains. FEMS Yeast Res. 2014, 14, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Shanmugam, K.T.; Ingram, L.O. l-Malate production by metabolically engineered Escherichia coli. Appl. Environ. Microbiol. 2011, 77, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zelle, R.M.; Hulster, E.; van Winden, W.A.; de Waard, P.; Dijkema, C.; Winkler, A.A.; Geertman, J.M.A.; van Dijken, J.P.; Pronk, J.T.; van Maris, A.J. Malic acid production by Saccharomyces cerevisiae: Engineering of pyruvate carboxylation, oxaloacetate reduction, and malate export. Appl. Environ. Microbiol. 2008, 74, 2766–2777. [Google Scholar] [CrossRef] [PubMed]
- Peleg, Y.; Stieglitz, B.; Goldberg, I. Malic acid accumulation by Aspergillus flavus. I. Biochemical aspects of acid biosynthesis. Appl. Microbiol. Biotechnol. 1988, 28, 69–75. [Google Scholar]
- Peleg, Y.; Barak, A.; Scrutton, M.C.; Goldberg, I. Malic acid accumulation by Aspergillus flavus. III. 13C NMR and isoenzymes analyses. Appl. Microbiol. Biotechnol. 1989, 30, 176–183. [Google Scholar] [CrossRef]
- Battat, E.; Peleg, Y.; Bercowitz, A.; Rokem, J.S.; Goldberg, I. Optimization of l-malic acid production by Aspergillus flavus in a stirred fermentor. Biotechnol. Bioeng. 1991, 37, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Bercovitz, A.; Peleg, Y; Battat, E.; Rokem, J.S.; Goldberg, I. Localization of pyruvate carboxylase in organic acid producing Aspergillus strains. Appl. Environ. Microbiol. 1990, 56, 1594–1597. [Google Scholar] [PubMed]
- Knuf, C.; Nookaew, I.; Brown, S.; McCulloch, M.; Berry, A.; Nielsen, J. Investigation of malic acid production in Aspergillus oryzae under nitrogen starvation conditions. Appl. Environ. Microbiol. 2013, 79, 6050–6058. [Google Scholar] [CrossRef] [PubMed]
- Ochsenreither, K.; Fischer, C.; Neumann, A.; Syldatk, C. Process characterization and influence of alternative carbon source and carbon-to-nitrogen ratio on organic acid production by Aspergillus oryzae DSM1863. Appl. Microbiol. Biotechnol. 2014, 98, 5449–5460. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, Y.; Zhang, H.; Wang, H.; Wu, Y.; Zhang, M.; Sun, T.; Cheng, J.; Wu, X.; Pan, L.; Jiang, S.; Wu, H. High levels of malic acid production by the conversion of corn straw hydrolyte using an isolated Rhizopus Delemar strain. Biotechnol. Bioprocess Eng. 2014, 19, 478–492. [Google Scholar] [CrossRef]
- Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. High-level production of calcium malate from glucose by Penicillium sclerotium K302. Bioresour. Technol. 2013, 143, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Knuf, C.; Nookaew, I.; Remmers, I.; Khoomrung, S.; Brown, S.; Berry, A.; Nielsen, J. Physiological characterization of the high malic acid-producing Aspergillus oryzae strain 21031-68. Appl. Microbiol. Biotechnol. 2014, 98, 3517–3527. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, G.; Xu, N.; Zou, W.; Zhu, P.; Liu, Liming; Chen, J. Metabolic engineering of Torulopsis glabrata for malate production. Metab. Eng. 2013, 19, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Mosier, N.S.; Hendrickson, R.; Ezeji, T.; Blaschek, H.; Dien, B.; Cotta, M.; Dale, B.; Ladisch, M.R. Composition of corn dry-grind ethanol by-products: DDGS, wet cake, and thin stillage. Bioresour. Technol. 2008, 99, 5165–5176. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Campbell, P.; Wong, M. Production of ethanol from thin stillage by metabolically engineered Escherichia coli. Biotechnol. Lett. 2010, 32, 405–411. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Malic acid production from thin stillage by Aspergillus species. Biotechnol. Lett. 2011, 33, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Tapasvi, D.; Wiesenborn, D.; Gustafson, C. Process model for biodiesel production from various feedstocks. Trans. ASAE 2005, 48, 2215–2221. [Google Scholar] [CrossRef]
- West, T.P. Crude glycerol: A feedstock for organic acid production by microbial bioconversion. J. Microb. Biochem. Technol. 2012. [Google Scholar] [CrossRef]
- Quispe, C.A.G; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—A byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Dobson, R.; Gray, V.; Rumbold, K. Microbial utilization of crude glycerol for the production of value-added products. J. Ind. Microbiol. Biotechnol. 2012, 39, 217–226. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Fungal biotransformation of crude glycerol into malic acid. Z. Naturforsch. C 2015, 70, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Kleineberg, W.; Sarikaya, E.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.B. Enhanced malic acid production from glycerol with high-density Ustilago trichophora TZ1 cultivations. Biotechnol. Biofuels 2016, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Sarikaya, E.; Kleineberg, W.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.B. Efficient malic acid production from glycerol with Ustilago trichophora TZ1. Biotechnol. Biofuels 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Oswald, F.; Dorsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential mixed cultures: From syngas to malic acid. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dorsam, S.; Kirchhoff, J.; Bigalke, M.; Dahmen, N.; Syldatk, C.; Ochsenreither, K. Evaluation of pyrolysis oil as carbon source for fungal fermentation. Front. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P. Reviving the carbohydrate economy via multi-product lignocellulose biorefineries. J. Ind. Microbiol. Biotechnol. 2008, 35, 367–375. [Google Scholar] [CrossRef] [PubMed]
- West, T.P. Xylitol production by Candida species grown on a grass hydrolysate. World J. Microbiol. Biotechnol. 2009, 25, 913–916. [Google Scholar] [CrossRef]
- West, T.P. Effect of nitrogen source concentration on curdlan production by Agrobacterium sp. ATCC 31749 grown on prairie cordgrass hydrolysates. Prep. Biochem. Biotechnol. 2016, 46, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lin, J.; Mao, Y.; Zhang, X. Systematic analysis of an evolved Thermobifida fusca muC producing malic acid on organic and inorganic nitrogen sources. Sci. Rep. 2016, 6, 30025. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Yang, J.; Tian, X.; Guo, M.; Li, Z.; Li, Y. Production of polymalic acid and malic acid from xylose and corncob hydrolysate by a novel Aureobasidium pullulans YJ 6-11 strain. Process Biochem. 2016, 51, 16–23. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Zhang, Y.; Wang, B.; Zou, X. Effects of nitrogen availability on polymalic acid biosynthesis in the yeast-like fungus Aureobasidium pullulans. Microb. Cell Fact. 2016, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Zan, Z.; Zou, X. Efficient production of polymalic acid from raw sweet potato hydrolysate with immobilized cells of Aureobasidium pullulans CCTC M2012223 in aerobic fibrous bed bioreactor. J. Chem. Technol. Biotechnol. 2013, 88, 1822–1827. [Google Scholar] [CrossRef]
- Wei, P.; Cheng, C.; Lin, M.; Zhou, Y.; Yang, S.-T. Production of poly(malic acid) from sugarcane juice in fermentation by Aureobasidium pullulans: Kinetics and process economics. Bioresour. Technol. 2017, 224, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.-T. Polymalic acid fermentation from Aureobasidium pullulans for malic acid production from soybean hull and soy molasses: Fermentation kinetics and economic analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]

| Coproduct/Hydrolyzed Lignocellulosic Biomass | Microorganism | Growth Conditions | Malic Acid (g/L) | Yield (g/g) | Reference |
|---|---|---|---|---|---|
| Corn stover | T. fusca muC-16 | 55 °C | 21.5 | 0.43 | [4] |
| Corn straw | R. delemar HF-119 | 30 °C | 60.0 | 0.48 | [22] |
| R. delemar HF-121 | 30 °C | 121.8 | 0.97 | [22] | |
| Thin stillage | A. flavus ATCC 13697 | 25 °C | 10.2 | 0.48 | [28] |
| A. niger ATCC 9029 | 25 °C | 1.0 | 0.05 | [28] | |
| A. niger ATCC 9142 | 25 °C | 16.9 | 0.79 | [28] | |
| A. niger ATCC 10577 | 25 °C | 16.4 | 0.79 | [28] | |
| Crude glycerol | A. niger ATCC 9142 | 25 °C | 16.5 | 0.171 | [34] |
| A. niger ATCC 10577 | 25 °C | 20.3 | 0.201 | [34] | |
| A. niger ATCC 12846 | 25 °C | 23.5 | 0.241 | [34] | |
| U. trichophora TZ1 | 30 °C | 108.0 | 0.26 | [35] | |
| Syngas (plant biomass) | C. ljungdahli DSM 13528/A. oryzae DSM 1863 | 25 °C | 1.1 | 0.17 | [37] |
| Biooil (plant biomass) | A. oryzae DSM 1863 | 32 °C | 0.0 | 0.0 | [38] |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
West, T.P. Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass. Fermentation 2017, 3, 14. https://doi.org/10.3390/fermentation3020014
West TP. Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass. Fermentation. 2017; 3(2):14. https://doi.org/10.3390/fermentation3020014
Chicago/Turabian StyleWest, Thomas P. 2017. "Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass" Fermentation 3, no. 2: 14. https://doi.org/10.3390/fermentation3020014
APA StyleWest, T. P. (2017). Microbial Production of Malic Acid from Biofuel-Related Coproducts and Biomass. Fermentation, 3(2), 14. https://doi.org/10.3390/fermentation3020014