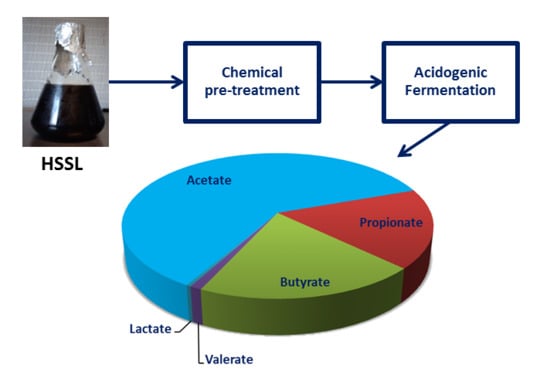
Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Culture
2.2. Experimental Setup
2.3. Substrate
2.4. Fermentation Medium
2.5. Sampling
2.6. Analytical Methods
2.7. Calculations
3. Results and Discussion
3.1. Acidogenic Fermentation of HSSL
3.2. Short-chain Organic Acids (SCOAs) Production
3.3. Acidification Degree
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fava, F.; Totaro, G.; Diels, L.; Reis, M.; Duarte, J.; Carioca, O.B.; Poggi-Varaldo, H.M.; Ferreira, B.S. Biowaste biorefinery in Europe: Opportunities and research & development needs. New Biotechnol. 2015, 32, 100–108. [Google Scholar]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Lopez Garcia, I.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Frison, N.; Katsou, E.; Malamis, S.; Oehmen, A.; Fatone, F. Development of a novel process integrating the treatment of sludge reject water and the production of polyhydroxyalkanoates (PHAs). Environ. Sci. Technol. 2015, 49, 10877–10885. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hu, H.; Ji, H.; Cai, J.; He, N.; Li, Q.; Wang, Y. Production of poly(hydroxybutyrate-hydroxyvalerate) from waste organics by the two-stage process: Focus on the intermediate volatile fatty acids. Bioresour. Technol. 2014, 166, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Woo Park, G.; Fei, Q.; Jung, K.; Chang, H.N.; Kim, Y.-C.; Kim, N.; Choi, J.; Kim, S.; Cho, J. Volatile fatty acids derived from waste organics provide an economical carbon source for microbial lipids/biodiesel production. Biotechnol. J. 2014, 9, 1536–1546. [Google Scholar] [CrossRef] [PubMed]
- Elefsiniotis, P.; Wareham, D.G.; Smith, M.O. Use of volatile fatty acids from an acid-phase digester for denitrification. J. Biotechnol. 2004, 114, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.C.; Serafim, L.S.; Nadais, H.; Arroja, L.; Capela, I. Acidogenic fermentation towards valorisation of organic waste streams into volatile fatty acids. Chem. Biochem. Eng. Q. 2013, 27, 467–476. [Google Scholar]
- Ke, S.; Shi, Z.; Fang, H.H.P. Applications of two-phase anaerobic degradation in industrial wastewater treatment. Int. J. Environ. Pollut. 2005, 23, 65–80. [Google Scholar] [CrossRef]
- Temudo, M.F.; Mato, T.; Kleerebezem, R.; van Loosdrecht, M.C.M. Xylose anaerobic conversion by open-mixed cultures. Appl. Microbiol. Biotechnol. 2009, 82, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Visvanathan, C.; Abeynayaka, A. Developments and future potentials of anaerobic membrane bioreactors (AnMBRs). Membr. Water Treat. 2012, 3, 1–23. [Google Scholar] [CrossRef]
- De Aquino, S.F.; Chernicharo, C.A.L. Acúmulo de ácidos graxos voláteis (AGVs) em reatores anaeróbios sob estresse: causas e estratégias de controle. Eng. Sanit. E Ambient. 2005, 10, 152–161. [Google Scholar] [CrossRef]
- Queirós, D.; Rossetti, S.; Serafim, L.S. PHA production by mixed cultures: A way to valorize wastes from pulp industry. Bioresour. Technol. 2014, 157, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Queirós, D.; Fonseca, A.; Lemos, P.C.; Serafim, L.S. Long-term operation of a two-stage polyhydroxyalkanoates production process from hardwood sulphite spent liquor. J. Chem. Technol. Biotechnol. 2016, 91, 2480–2487. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Scoma, A.; Bertin, L.; Fava, F. Effect of hydraulic retention time on biohydrogen and volatile fatty acids production during acidogenic digestion of dephenolized olive mill wastewaters. Biomass Bioenergy 2013, 48, 51–58. [Google Scholar] [CrossRef]
- Miron, Y.; Zeeman, G.; van Lier, J.B.; Lettinga, G. The role of sludge retention time in the hydrolysis and acidification of lipids, carbohydrates and proteins during digestion of primary sludge in CSTR systems. Water Res. 2000, 34, 1705–1713. [Google Scholar] [CrossRef]
- Dogan, E.; Demirer, G.N. Volatile fatty acid production from organic fraction of municipal solid waste through anaerobic acidogenic digestion. Environ. Eng. Sci. 2009, 26, 1443–1450. [Google Scholar] [CrossRef]
- Rueda, C.; Calvo, P.A.; Moncalián, G.; Ruiza, G.; Coz, A. Biorefinery options to valorize the spent liquor from sulfite pulping. J. Chem. Technol. Biotechnol. 2015, 90, 2218–2226. [Google Scholar] [CrossRef]
- Zygmunt, B.; Banel, A. Formation, occurrence and determination of volatile fatty acids in environmental and related samples. In Proceedings of the 3rd WSEAS International Conference on Energy Planning, Energy Saving, Environmental Education, Renewable Energy Sources, Waste Management, Tenerife, Spain, 2009; pp. 476–481. [Google Scholar]
- Pereira, S.R.; Ivanuša, S.; Evtuguin, D.V; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, 1998; Available online: https://www.standardmethods.org/ (accessed on 1 March 2015).
- Restolho, J.A.; Prates, A.; de Pinho, M.N.; Afonso, M.D. Sugars and lignosulphonates recovery from eucalyptus spent sulphite liquor by membrane processes. Biomass Bioenergy 2009, 33, 1558–1566. [Google Scholar] [CrossRef]
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Morales, F.J.; Villaseñor, J.; Infantes, D. Modeling and monitoring of the acclimatization of conventional activated sludge to a biohydrogen producing culture by biokinetic control. Int. J. Hydrog. Energy 2010, 35, 10927–10933. [Google Scholar] [CrossRef]
- Jeffries, T.W. Advances in biochemical engineering/biotechnology. In Pentoses and Lignin; Fieehter, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 1–32. [Google Scholar]
- Prakasham, R.S.; Brahmaiah, P.; Sathish, T.; Sambasiva Rao, K.R.S. Fermentative biohydrogen production by mixed anaerobic consortia: Impact of glucose to xylose ratio. Int. J. Hydrog. Energy 2009, 34, 9354–9361. [Google Scholar] [CrossRef]
- Bengtsson, S.; Hallquist, J.; Werker, A.; Welander, T. Acidogenic fermentation of industrial wastewaters: Effects of chemostat retention time and pH on volatile fatty acids production. Biochem. Eng. J. 2008, 40, 492–499. [Google Scholar] [CrossRef]
- Jie, W.; Peng, Y.; Ren, N.; Li, B. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour. Technol. 2014, 152, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Gemert, J.M.V.; Zoetemeyer, R.J.; Breure, A.M. Main characteristics and stoichiometric aspects of acidogenesis of soluble carbohydrate containing wastewater. Process Biochem. 1984, 19, 282–286. [Google Scholar]
- Chang, H.N.; Kim, N.-J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Horiuchi, J.-I.; Shimizu, T.; Tada, K.; Kanno, T.; Kobayashi, M. Selective production of organic acids in anaerobic acid reactor by pH control. Bioresour. Technol. 2002, 82, 209–213. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, B.J.; Jeong, C.M.; Choi, J.D.; Ahn, Y.H.; Chang, H.N. Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.R.; Freitas, E.B.; Galinha, C.F.; Carvalho, G.; Duque, A.F.; Reis, M.A. Dynamic change of pH in acidogenic fermentation of cheese whey towards polyhydroxyalkanoates production: Impact on performance and microbial population. New Biotechnol. 2017, 37, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Tada, K.; Kanno, T.; Horiuchi, J.I. Selective production of lactic acid in continuous anaerobic acidogenesis by extremely low pH operation. J. Biosci. Bioeng. 2012, 114, 537–539. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.G.E.; Eiroa, M.; Torres, C.; Nunes, B.R.; Reis, M.A.M. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007, 130, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Jantsch, T.G.; Angelidaki, I.; Schmidt, J.E.; Braña de Hvidsten, B.E.; Ahring, B.K. Anaerobic biodegradation of spent sulphite liquor in a UASB reactor. Biores. Technol. 2002, 84, 15–20. [Google Scholar] [CrossRef]
- Pereira, S.R.; Portugal-Nunes, D.J.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Advances in ethanol production from hardwood spent sulphite liquors. Process Biochem. 2013, 48, 272–282. [Google Scholar] [CrossRef]



| Parameters | Time (Day) | Sugars Consumed * | SCOAs * (g COD/L) | SCOAs Profile (% COD) * | AD * (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| g COD/L | (%) | Lactate | Acetate | Propionate | Butyrate | Valerate | ||||
| Operation | 0–88 | 3.8 ± 0.29 | 94 ± 7.2 | 4.0 ± 1.76 | 3.9 ± 3.91 | 59 ± 6.8 | 17 ± 6.8 | 19 ± 6.5 | 1.0 ± 1.3 | 20 ± 9.0 |
| PSS | 46–88 | 3.8 ± 0.25 | 94 ± 6.3 | 5.5 ± 0.84 | 5.7 ± 3.91 | 53 ± 3.2 | 22 ± 4.2 | 19 ± 7.5 | 0.0 ± 0.00 | 28 ± 4.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queirós, D.; Sousa, R.; Pereira, S.; Serafim, L.S. Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids. Fermentation 2017, 3, 20. https://doi.org/10.3390/fermentation3020020
Queirós D, Sousa R, Pereira S, Serafim LS. Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids. Fermentation. 2017; 3(2):20. https://doi.org/10.3390/fermentation3020020
Chicago/Turabian StyleQueirós, Diogo, Rita Sousa, Susana Pereira, and Luísa S. Serafim. 2017. "Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids" Fermentation 3, no. 2: 20. https://doi.org/10.3390/fermentation3020020
APA StyleQueirós, D., Sousa, R., Pereira, S., & Serafim, L. S. (2017). Valorization of a Pulp Industry By-Product through the Production of Short-Chain Organic Acids. Fermentation, 3(2), 20. https://doi.org/10.3390/fermentation3020020