Tuning of the Carbon-to-Nitrogen Ratio for the Production of l-Arginine by Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Seed Cultures for Fermentations
2.3. Fermentations
2.4. Cell Growth Analyses
2.5. Substrates, Products and By-Products Analyses
3. Results and Discussion
3.1. Comparison of Nitrogen Sources
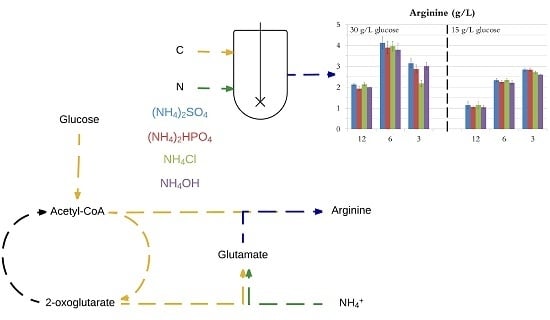
3.2. Comparison of C/N Ratios
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loscalzo, J. l-arginine and atherothrombosis. J. Nutr. 2004, 134, 2798S–2800S; discussion 2818S–2819S. [Google Scholar] [PubMed]
- Öhlund, J.; Näsholm, T. Low nitrogen losses with a new source of nitrogen for cultivation of conifer seedlings. Environ. Sci. Technol. 2002, 36, 4854–4859. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, X.; Wan, F.; Zhang, B.; Chen, J.; Xiong, Y. Effect of Tween 40 and DtsR1 on l-arginine overproduction in Corynebacterium crenatum. Microb. Cell Fact. 2015, 14, 119. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Yoshida, H. Fermentative production of l-arginine. Agric. Biol. Chem. 1972, 36, 1675–1684. [Google Scholar] [CrossRef]
- Dou, W.; Xu, M.; Cai, D.; Zhang, X.; Rao, Z.; Xu, Z. Improvement of l-arginine production by overexpression of a bifunctional ornithine acetyltransferase in Corynebacterium crenatum. Appl. Biochem. Biotechnol. 2011, 165, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, H.U.; Kim, T.Y.; Park, J.S.; Kim, S.; Lee, S.Y. Metabolic engineering of Corynebacterium glutamicum for l-arginine production. Nat. Commun. 2014, 5, 4618. [Google Scholar] [CrossRef] [PubMed]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Ghosh, D.; Hallenbeck, P.C. Fermentative hydrogen yields from different sugars by batch cultures of metabolically engineered Escherichia coli DJT135. Int. J. Hydrog. Energy 2009, 34, 7979–7982. [Google Scholar] [CrossRef]
- Dien, B.S.; Nichols, N.N.; Bothast, R.J. Recombinant Escherichia coli engineered for production of l-lactic acid from hexose and pentose sugars. J. Ind. Microbiol. Biotechnol. 2001, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Hodge, D.; Berglund, K.A.; Rova, U. Effect of different carbon sources on the production of succinic acid using metabolically engineered Escherichia coli. Biotechnol. Prog. 2007, 23, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Ginesy, M.; Belotserkovsky, J.; Enman, J.; Isaksson, L.; Rova, U. Metabolic engineering of Escherichia coli for enhanced arginine biosynthesis. Microb. Cell Fact. 2015, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Neidhardt, F.C.; Ingraham, J.L.; Low, K.B.; Magasanik, B.; Schaechter, M.; Umbarger, H. Escherichia coli and Salmonella Typhimurium: Cellular and Molecular Biology; American Society for Microbiology: Washington, DC, USA, 1987; Volume 2. [Google Scholar]
- Haleem Shah, A.; Hameed, A.; Ahmad, S.; Majid Khan, G. Optimization of culture conditions for l-lysine fermentation by Corynebacterium glutamicum. J. Biol. Sci. 2002, 2, 151–156. [Google Scholar]
- Jeon, J.M.; Rajesh, T.; Song, E.; Lee, H.W.; Lee, H.W.; Yang, Y.H. Media optimization of Corynebacterium glutamicum for succinate production under oxygen-deprived condition. J. Microbiol. Biotechnol. 2013, 23, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Huang, J.; Feng, Z.B.; Yu, L.; Xu, Q.Y.; Wen, T.Y. Optimization of fermentation conditions for the biosynthesis of l-threonine by Escherichia coli. Appl. Biochem. Biotechnol. 2009, 158, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Cao, W.; Wang, Z.; Chen, K.; Li, Y.; Ouyang, P. Enhancement of l-phenylalanine production by engineered Escherichia coli using phased exponential l-tyrosine feeding combined with nitrogen source optimization. J. Biosci. Bioeng. 2015, 120, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Hahn, J.; Crutzen, P.J. The role of fixed nitrogen in atmospheric photochemistry. Philos. Trans. R. Soc. Lond. Ser. B 1982, 296, 521–541. [Google Scholar] [CrossRef]
- Kinzig, A.P.; Socolow, R.H. Human impacts on the nitrogen cycle. Physics Today 1994, 47, 24–31. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Galloway, J.N. The global nitrogen cycle: Changes and consequences. Environ. Pollut. 1998, 102, 15–24. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Boucher, O.; Haigh, J.; Hauglustaine, D.; Haywood, J.; Myhre, G.; Nakajima, T.; Shi, G.; Solomon, S.; Betts, R.E.; et al. Radiative Forcing of Climate Change; Houghton, J.T., Callander, B.A., Varney, S.K., Eds.; Cambridge University Press: New York, NY, USA, 2001. [Google Scholar]
- Dioha, I.; Ikeme, C.; Nafi’u, T.; Soba, N. Effect of carbon to nitrogen ratio on biogas production. IRJNS 2013, 1, 1–10. [Google Scholar]
- Wu, X.; Yao, W.; Zhu, J.; Miller, C. Biogas and CH4 productivity by co-digesting swine manure with three crop residues as an external carbon source. Bioresour. Technol. 2010, 101, 4042–4047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Sattur, A.P.; Karanth, N.G. Production of microbial lipids: II. Influence of C/N ratio—model prediction. Biotechnol. Bioeng. 1989, 34, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Ykema, A.; Verbree, E.C.; Kater, M.M.; Smit, H. Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in wheypermeate. Appl. Microbiol. Biotechnol. 1988, 29, 211–218. [Google Scholar] [CrossRef]
- Kalil, M.S.; Alshiyab, H.S.; Yusoff, W.M.W. Effect of nitrogen source and carbon to nitrogen ratio on hydrogen production using C. acetobutylicum. Am. J. Biochem. Biotechnol. 2008, 4, 393–401. [Google Scholar] [CrossRef]
- Wang, D.; Wei, G.; Nie, M.; Chen, J. Effects of nitrogen source and carbon/nitrogen ratio on batch fermentation of glutathione by Candida utilis. Korean J. Chem. Eng. 2010, 27, 551–559. [Google Scholar] [CrossRef]
- Kumar, R.; Shimizu, K. Metabolic regulation of Escherichia coli and its gdhA, glnL, gltB D mutants under different carbon and nitrogen limitations in the continuous culture. Microb. Cell Fact. 2010, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Mohd Rusli, F.; Mohamed, M.S.; Mohamed, R.; Puspaningsih, N.N.T.; Ariff, A. Kinetics of xylanase fermentation by recombinant Escherichia coli DH5a in shake flask culture. Am. J. Biochem. Biotechnol. 2009, 5, 110–118. [Google Scholar]
- Commichau, F.M.; Forchhammer, K.; Stülke, J. Regulatory links between carbon and nitrogen metabolism. Microb. Cell Fact. 2006, 9, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.J.; Huo, Y.X.; Buck, M.; Kolb, A.; Wang, Y.P. Interplay between CRP-cAMP and PII-Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucleic Acids Res. 2007, 35, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Doucette, C.D.; Schwab, D.J.; Wingreen, N.S.; Rabinowitz, J.D. alpha-ketoglutarate coordinates carbon and nitrogen utilization via Enzyme I inhibition. Nat. Chem. Biol. 2011, 7, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Helling, R.B. Pathway choice in glutamate synthesis in Escherichia coli. J. Bacteriol. 1998, 180, 4571–4575. [Google Scholar] [PubMed]
- Nakano, M.; Iida, T.; Ohnishi, M.; Kurokawa, K.; Takahashi, A.; Tsukamoto, T.; Yasunaga, T.; Hayashi, T.; Honda, T. Association of the urease gene with enterohemorrhagic Escherichia coli strains irrespective of their serogroups. J. Clin. Microbiol. 2001, 39, 4541–4543. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Charusanti, P.; Aziz, R.K.; Lerman, J.A.; Premyodhin, N.; Orth, J.D.; Feist, A.M.; Palsson, B.Ø. Genome-scale metabolic reconstructions of multiple Escherichia coli strains highlight strain-specific adaptations to nutritional environments. Proc. Natl. Acad. Sci. USA 2013, 110, 20338–20343. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Ishimoto, M. Aerobic inhibition of nitrate assimilation in Escherichia coli. Z. Allg. Mikrobiol. 1973, 13, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bren, A.; Park, J.O.; Towbin, B.D.; Dekel, E.; Rabinowitz, J.D.; Alon, U. Glucose becomes one of the worst carbon sources for E. coli on poor nitrogen sources due to suboptimal levels of cAMP. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gage, D.; Zhan, J. Efficient production of indigoidine in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2015, 42, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Luli, G.W.; Strohl, W.R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl. Environ. Microbiol. 1990, 56, 1004–1011. [Google Scholar] [PubMed]
- Eiteman, M.A.; Altman, E. Overcoming acetate in Escherichia coli recombinant protein fermentations. Trends Biotechnol. 2006, 24, 530–536. [Google Scholar] [CrossRef] [PubMed]




| Nitrogen Source | μ (1/h) | DCW (g/L) | l-arginine (g/L) | Qp (g/L/h) |
|---|---|---|---|---|
| Ammonia solution | 0.10 ± 0.01 | 2.35 ± 0.08 | 2.30 ± 0.09 | 0.12 ± 0.01 |
| Ammonium carbonate | 0.06 * | 2.08 ± 0.02 | 2.11 ± 0.01 | 0.08 * |
| Ammonium chloride | 0.09 * | 2.18 ± 0.03 | 2.23 ± 0.06 | 0.12 ± 0.01 |
| Ammonium nitrate | 0.06 * | 2.31 ± 0.12 | 1.44 ± 0.11 | 0.05 * |
| Ammonium phosphate dibasic | 0.09 * | 2.25 ± 0.03 | 2.25 ± 0.06 | 0.13 ± 0.02 |
| Ammonium sulphate | 0.09 ± 0.01 | 2.30 ± 0.10 | 2.41 ± 0.08 | 0.13 ± 0.01 |
| Sodium nitrate | nd | nd | nd | nd |
| Monosodium glutamate 10 g/L | nd | nd | nd | nd |
| Monosodium glutamate 5 g/L | nd | nd | nd | nd |
| Monosodium glutamate 2.5 g/L | 0.01 * | 0.52 ± 0.02 | nd | nd |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ginésy, M.; Rusanova-Naydenova, D.; Rova, U. Tuning of the Carbon-to-Nitrogen Ratio for the Production of l-Arginine by Escherichia coli. Fermentation 2017, 3, 60. https://doi.org/10.3390/fermentation3040060
Ginésy M, Rusanova-Naydenova D, Rova U. Tuning of the Carbon-to-Nitrogen Ratio for the Production of l-Arginine by Escherichia coli. Fermentation. 2017; 3(4):60. https://doi.org/10.3390/fermentation3040060
Chicago/Turabian StyleGinésy, Mireille, Daniela Rusanova-Naydenova, and Ulrika Rova. 2017. "Tuning of the Carbon-to-Nitrogen Ratio for the Production of l-Arginine by Escherichia coli" Fermentation 3, no. 4: 60. https://doi.org/10.3390/fermentation3040060
APA StyleGinésy, M., Rusanova-Naydenova, D., & Rova, U. (2017). Tuning of the Carbon-to-Nitrogen Ratio for the Production of l-Arginine by Escherichia coli. Fermentation, 3(4), 60. https://doi.org/10.3390/fermentation3040060