Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation
Abstract
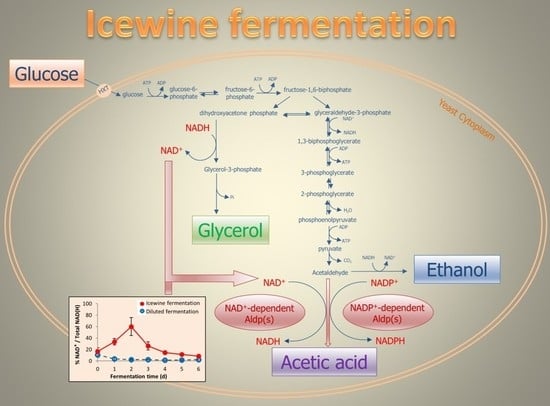
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Juices
2.2. Fermentation Setup and Sampling
2.3. Analysis for Metabolites
2.4. Analysis for Enzyme Cofactors
2.5. Statistical Analysis
3. Results
3.1. Fermentation Kinetics
3.2. Yeast Metabolite Production
3.3. Ratio of Coenzyme Concentration
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bowen, A. Managing the quality of icewines. In Managing Wine Quality: Oenology and Wine Quality, 1st ed.; Reynolds, A.G., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2010; pp. 523–552. [Google Scholar]
- O. Reg. 406/00: Rules of Vintners Quality Alliance Ontario Relating to Terms for VQA Wine. Available online: https://www.ontario.ca/laws/regulation/000406 (accessed on 14 November 2017).
- Ziraldo, D.; Kaiser, K. Science. In Icewine: Extreme Winemaking, 1st ed.; Key Porter Books: Toronto, ON, Canada, 2007; pp. 73–107. [Google Scholar]
- Kontkanen, D.; Inglis, D.; Pickering, G.; Reynolds, A. Effect of yeast inoculation rate, acclimatization, and nutrient addition on Icewine fermentation. Am. J. Enol. Vitic. 2004, 55, 363–370. [Google Scholar]
- Nurgel, C.; Pickering, G.J.; Inglis, D.L. Sensory and chemical characteristics of Canadian ice wines. J. Sci. Food Agric. 2004, 84, 1675–1684. [Google Scholar] [CrossRef]
- Cliff, M.A.; Pickering, G.J. Determination of odour detection thresholds for acetic acid and ethyl acetate in ice wine. J. Wine Res. 2006, 17, 45–52. [Google Scholar] [CrossRef]
- Lilly, M.; Lambrechts, M.G.; Pretorius, I.S. Effect of increased acetyltransferase activity on flavour profiles of wine and distillates. Appl. Environ. Microbiol. 2000, 66, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, A.; Adler, L. Roles of glycerol and glycerol-3-phosphate dehydrogenase (NAD+) in acquired osmotolerance of Saccharomyces cerevisiae. J. Bacteriol. 1989, 171, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, R.; Ganedo, J.M. Reduced pyridine nucleotide balance growing on Saccharomyces cerevisiae. Eur. J. Biochem. 1973, 37, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Van Dijken, J.; Scheffers, W. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Rev. 1986, 32, 199–224. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Inglis, D.L. Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during Icewine fermentation. J. Appl. Microbiol. 2005, 99, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Pigeau, G.M.; Inglis, D.L. Response of wine yeast (Saccharomyces cerevisiae) aldehyde dehydrogenases to acetaldehyde stress during Icewine fermentation. J. Appl. Microbiol. 2007, 103, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Heit, C.; Martin, S.J.; Yang, F.; Inglis, D.L. Osmoadaptation of wine yeast (Saccharomyces cerevisiae) to hyperosmotic stress during Icewine fermentation. J. Appl. Microbiol. 2017. under review. [Google Scholar]
- Pronk, J.T.; Steensma, H.Y.; van Dijken, J.P. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast 1996, 12, 1607–1633. [Google Scholar] [CrossRef]
- Erasmus, D.J.; van Vuuren, H.J.J. Genetic basis for osmosensitivity and genetic instability of the wine yeast Saccharomyces cerevisiae V1N7. Am. J. Enol. Vitic. 2009, 60, 145–154. [Google Scholar]
- Navarro-Avino, J.P.; Prasad, R.; Miralles, V.J.; Benito, R.M.; Serreno, R. A proposal of nomenclature of aldehyde dehydrogenases in Saccharomyces cerevisiae and characterization of the stress-inducible ALD2 and ALD3 genes. Yeast 1999, 15, 829–842. [Google Scholar] [CrossRef]
- Meaden, P.G.; Dickinson, F.M.; Mifsud, A.; Tessier, W.; Westwater, J.; Bussey, H.; Midgley, M. The ALD6 gene of Saccharomyces cerevisiae encodes a cytosolic, Mg2+-activated acetaldehyde dehydrogenase. Yeast 1997, 13, 1319–1327. [Google Scholar] [CrossRef]
- White, H.W.; Skatrud, P.L.; Xue, Z.; Toyn, J.H. Specialization of function among aldehyde dehydrogenases: The ALD2 and ALD3 genes are required for B-alanine biosynthesis in S. cerevisiae. Genetics 2003, 163, 69–77. [Google Scholar] [PubMed]
- Eglinton, J.M.; Heinrich, A.J.; Pollnitz, A.P.; Langridge, P.; Henschke, P.A.; de Barros Lopes, M. Decreasing acetic acid accumulation by a glycerol overproducing strain of Saccharomyces cerevisiae by deleting the ALD6 aldehyde dehydrogenase gene. Yeast 2002, 19, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Walkey, C.; Madilao, L.; Measday, V.; Van Vuuren, H. Functional improvement of Saccharomyces cerevisiae to reduce volatile acidity in wine. FEMS Yeast Res. 2013, 13, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Noti, O.; Vaudano, E.; Pessione, E.; Garcia-Moruno, E. Short-term response of different Saccharomyces cerevisiae strains to hyperosmotic stress caused by inoculation in grape must: RT-qPCR study and metabolite analysis. Food Microbiol. 2015, 52, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Saint-Prix, F.; Bönquist, L.; Dequin, S. Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: The NADP+-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology 2004, 150, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Remize, F.; Andrieu, E.; Dequin, S. Engineering of the pyruvate dehydrogenase bypass in Saccharomyces cerevisiae: Role of the cytosolic Mg2+ and mitochondrial K+ acetaldehyde dehydrogenases Ald6p and Ald4p in acetate formation during alcoholic fermentation. Appl. Environ. Microbiol. 2000, 66, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, S.; Kamiya, H.; Yamashita, N.; Motoyoshi, T.; Goto-Yamamoto, N.; Ishikawa, T.; Okazaki, N.; Nishimura, A. Effects of aldehyde dehydrogenase and acetyl-CoA synthetase on acetate formation in sake mash. J. Biosci. Bioeng. 2000, 90, 555–560. [Google Scholar] [CrossRef]
- Forster, J.; Famili, I.; Fu, P.; Palsson, B.O.; Nielsen, J. Genome-scale reconstruction of Saccharomyces cerevisiae metabolic network. Genome Res. 2003, 13, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bruinenberg, P.M. The NADP(H) redox couple in yeast metabolism. Antonie Van Leeuwenhoek 1986, 52, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Martínez-Moreno, R.; Albiol, J.; Morales, P.; Vázquez-Lima, F.; Barreiro-Vázquez, A.; Ferrer, P.; Gonzalez, R. Metabolic flux analysis during the exponential growth phase of Saccharomyces cerevisiae in wine fermentations. PLoS ONE 2013, 8, e71909. [Google Scholar] [CrossRef]
- Villadsen, J.; Nielsen, J.; Lidén, G. Chemicals from metabolic pathways. In Bioreaction Engineering Principles, 3rd ed.; Springer: New York, NY, USA, 2011; pp. 7–62. [Google Scholar]
- Nissen, T.L.; Schulze, U.; Nielsen, J.; Villadsen, J. Flux distributions in anaerobic, glucose-limited continuous cultures of Saccharomyces cerevisiae. Microbiology 1997, 143, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Pizarro, F.; Agosin, E. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl. Environ. Microbiol. 2004, 70, 3392–3400. [Google Scholar] [CrossRef] [PubMed]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H. Laboratory Procedures. In Wine Analysis and Production, 1st ed.; Chapman and Hall, International Thomson Publishing: New York, NY, USA, 1995; pp. 374–378. [Google Scholar]




| Parameter | Icewine Juice | Diluted Juice |
|---|---|---|
| Glucose + fructose (g L−1) | 433 ± 5 | 201 ± 1 |
| Titratable acidity (g L−1) | 6.4 ± 0.0 | 3.1 ± 0.0 |
| pH | 3.76 ± 0.01 | 3.88 ± 0.01 |
| Free amino nitrogen (mg N L−1) | 238.4 ± 2.4 | 114.8 ± 3.0 |
| Ammonia nitrogen (mg N L−1) | 14.8 ± 0.1 | 6.6 ± 0.8 |
| Glycerol (g L−1) | 8.76 ± 0.06 | 4.04 ± 0 |
| Acetaldehyde (mg L−1) | 5.3 ± 0.8 | 2.7 ± 0.2 |
| Acetic acid (g L−1) | 0.04 ± 0 | 0.02 ± 0 |
| Ethanol (% v/v) | 1.3 ± 0 | 0.6 ± 0 |
| Parameter | Icewine Juice Fermentation | Diluted Juice Fermentation |
|---|---|---|
| Glucose + fructose (g L−1) | 252 ± 3 | 1 ± 1 |
| Titratable acidity (g L−1) | 8.3 ± 0.2 | 5.5 ± 0.1 |
| pH | 3.92 ± 0.01 | 3.63 ± 0.02 |
| Free amino nitrogen (mg N L−1) | 188.5 ± 1.4 | 7.4 ± 0.4 |
| Ammonia nitrogen (mg N L−1) | 3.5 ± 0.1 | † ND |
| Glycerol (g L−1) | 17.44 ± 0.19 | 9.43 ± 0.06 |
| Acetaldehyde (mg L−1) | 48.5 ± 0.8 | 23.0 ± 0.8 |
| Acetic acid (g L−1) | 1.48 ± 0.10 | 0.12 ± 0.01 |
| Ethanol (% v/v) | 12.0 ± 0.2 | 13.9 ± 0.1 |
| Fermentation | Glycerol (mg g−1 of Sugar Consumed) * | Acetaldehyde (mg g−1 of Sugar Consumed) * | Ethanol (g g−1 of Sugar Consumed) * | Acetic Acid (mg g−1 of Sugar Consumed) * |
|---|---|---|---|---|
| Diluted juice | 27.44 ± 0.30 | 0.23 ± 0.01 | 0.53 ± 0 | 0.49 ± 0.06 |
| Icewine Juice | 50.92 ± 0.74 | 0.10 ± 0 | 0.47 ± 0 | 8.10 ± 0.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Heit, C.; Inglis, D.L. Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation. Fermentation 2017, 3, 61. https://doi.org/10.3390/fermentation3040061
Yang F, Heit C, Inglis DL. Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation. Fermentation. 2017; 3(4):61. https://doi.org/10.3390/fermentation3040061
Chicago/Turabian StyleYang, Fei, Caitlin Heit, and Debra L. Inglis. 2017. "Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation" Fermentation 3, no. 4: 61. https://doi.org/10.3390/fermentation3040061
APA StyleYang, F., Heit, C., & Inglis, D. L. (2017). Cytosolic Redox Status of Wine Yeast (Saccharomyces Cerevisiae) under Hyperosmotic Stress during Icewine Fermentation. Fermentation, 3(4), 61. https://doi.org/10.3390/fermentation3040061