Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking
Abstract
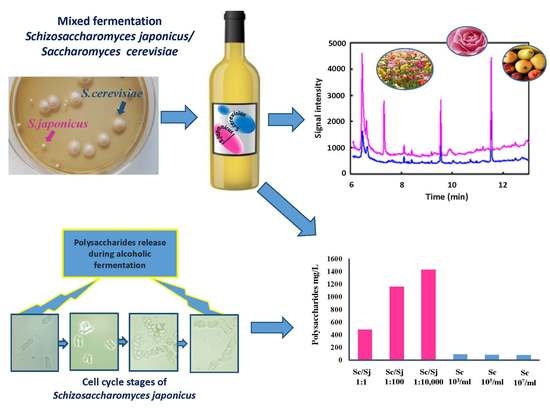
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Fermentation Trials
2.3. Growth Kinetics
2.4. Analytical Determinations of the Fermentation Products
2.5. Volatile Compound Analysis
2.6. Data Analysis
3. Results and Discussion
3.1. Cell Growth and Fermentation Kinetics
3.2. Metabolic Profile of Fermentations
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Fleet, G. Wine yeast for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Bisson, L.F.; Joseph, C.L.; Domizio, P. Yeasts. In Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Cham, Switzerland, 2017; pp. 65–101. [Google Scholar]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Rankine, B.C. Decomposition of l-malic acid by wine yeasts. J. Sci. Food Agric. 1966, 17, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M. Continuous deacidification of wine by immobilized Schizosaccharomyces pombe cells: Evaluation of malic acid degradation rate and analytical profiles. J. Appl. Microbiol. 1995, 79, 631–634. [Google Scholar]
- Silva, S.; Portugal, F.R.; Andrade, P.; Abreu, S.; Texeira, M.; Strehaiano, P. Malic acid consumption by dry immobilized cells of Schizosaccharomyces pombe. Am. J. Enol. Vitic. 2003, 54, 50–55. [Google Scholar]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combined use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed]
- Zara, G.; Mannazzu, I.; Del Caro, A.; Budroni, M.; Pinna, MB.; Murru, M.; Farris, G.A.; Zara, S. Wine quality improvement through the combined utilisation of yeast hulls and Candida zemplinina/Saccharomyces cerevisiae mixed starter cultures. Aust. J. Grape Wine Res. 2014, 20, 199–207. [Google Scholar] [CrossRef]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Use of non-Saccharomyces wine yeasts as novel sources of mannoproteins in wine. Food Microbiol. 2014, 43, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Munyon, J.R.; Nagel, C.W. Comparison of methods of deacidification of musts and wines. Am. J. Enol. Vitic. 1977, 28, 79–87. [Google Scholar]
- Snow, P.G.; Gallander, J.F. Deacidification of white table wines through partial fermentation with Schizosaccharomyces pombe. Am. J. Enol. Vitic. 1979, 30, 45–48. [Google Scholar]
- Magyar, I.; Panyik, I. Biological deacidification of wine with Schizosaccharomyces pombe Entrapped in Ca-alginate gel. Am. J. Enol. Vitic. 1989, 40, 233–240. [Google Scholar]
- Yokotsuka, K.; Otaki, A.; Naitoh, A.; Tanaka, H. Controlled simultaneous deacidification and alcohol fermentation of a high-acid grape must using two immobilized yeasts, Schizosaccharomyces pombe and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1993, 44, 371–377. [Google Scholar]
- Gao, C.; Fleet, G.H.H. Degradation of malic and tartaric-acids by high-density cell-suspensions of wine yeasts. Food Microbiol. 1995, 12, 65–71. [Google Scholar] [CrossRef]
- Thornton, R.J.; Rodriguez, S.B. Deacidification of red and white wines by a mutant of Schizosaccharomyces malidevorans under commercial winemaking conditions. Food Microbiol. 1996, 13, 475–482. [Google Scholar] [CrossRef]
- Dharmadhikari, M.R.; Wilker, K.L. Deacidification of high malate must with Schizosaccharomyces pombe. Am. J. Enol. Vitic. 1998, 49, 408–412. [Google Scholar]
- Morata, A.; Benito, S.; Loira, I.; Palomero, F.; González, M.C.; Suárez-Lepe, J.A. Formation of pyranoanthocyanins by Schizosaccharomyces pombe during the fermentation of red must. Int. J. Food Microbiol. 2012, 159, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. New applications 490 for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int. J. Food Sci. Technol. 2012, 47, 2101–2108. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol. 2014, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Manners, D.J.; Meyer, M.T. The molecular structure of some glucans from the cell walls of Schizosaccharomyces pombe. Carbohydr. Res. 1977, 57, 189–203. [Google Scholar] [CrossRef]
- Lubbers, S.; Léger, B.; Charpentier, C.; Feuillat, M. Effet colloïdes protecteurs d’extraits de parois de levures sur la stabilité tartrique d’un vin modèle. J. Int. Sci. Vigne Vin 1993, 27, 13–22. [Google Scholar]
- Waters, E.J.; Pellerin, P.; Brillouet, J.-M. A Saccharomyces mannoprotein that protects 619 wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Moine-Ledoux, V.; Dubourdieu, D. An invertase fragment responsible for improving the protein stability of dry white wines. J. Sci. Food Agric. 1999, 79, 537–543. [Google Scholar] [CrossRef]
- Dupin, I.V.; McKinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Williams, P.J.; Waters, E.J. Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal for their mechanism of action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Stockdale, V.J.; Pettolino, F.; Pocock, K.F.; De Barros Lopes, M.; Williams, P.J.; Bacic, A.; Fincher, G.B.; Høj, P.B.; Waters, E.J. Reducing haziness in white wine by overexpression of Saccharomyces cerevisiae genes YOL155c and YDR055w. Appl. Microb. Biotechnol. 2007, 73, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A recombinant Saccharomyces cerevisiae strain overproducing mannoproteins stabilizes wine against protein haze. Appl. Environ. Microbiol. 2008, 74, 5533–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeastsand the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailon, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast walls on the behavior of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 13–22. [Google Scholar]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Pallman, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Taillandier, P.; Gilis, M.; Strehaiano, P. Deacidification by Schizosaccharomyces: Interactions with Saccharomyces. J. Biotechnol. 1995, 40, 199–205. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma. A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Cheng, G.; Liu, Y.; Yue, T.-X.; Zhang, Z.-W. Comparison between aroma compounds in wines from four Vitis vinifera grape varieties grown in different shoot positions. Food Sci. Technol. 2015, 35, 237–246. [Google Scholar] [CrossRef]


| Yeasts | Inoculum Cell Concentration | pH | Total Acidity (g/L, Tartaric Acid) | Volatile Acidity (g/L, Acetic Acid) | Ethanol (%, v/v) | Sugar (%, w/v) | Malic Acid (g/L) | Glycerol (g/L) | Polysaccharides (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| S. cerevisiae | (107 cell/mL) | 3.35 ± 0.02 b | 6.60 ± 0.13 a | 0.36 ± 0.01 a | 13.78 ± 0.28 a | 0.19 ± 0.01 b | 1.30 ± 0.03 a | 6.57 ± 0.12 b | 88 ± 13.0 d |
| S. cerevisiae | (105 cell/mL) | 3.37 ± 0.02 b | 6.68 ± 0.00 a | 0.41 ± 0.03 a | 13.73 ± 0.26 a | 0.21 ± 001 b | 1.29 ± 0.05 a | 6.73 ± 0.35 b | 84 ± 3.0 d |
| S. cerevisiae | (103 cell/mL) | 3.33 ± 0.02 b | 6.62 ± 0.16 a | 0.44 ± 0.06 a | 13.62 ± 0.39 a | 0.21 ± 0.01 b | 1.20 ± 0.06 b | 7.21 ± 0.11 b | 80 ± 1.4 d |
| S. cerevisiae/S. japonicus | (107 cell/mL)/(107 cell/mL) Ratio 1:1 | 3.39 ± 0.04 b | 6.31 ± 0.04 a | 0.37 ± 0.04 a | 13.80 ± 0.24 a | 0.20 ± 0.01 b | 1.14 ± 0.03 b | 8.60 ± 0.83 b | 481 ± 26 c |
| S. cerevisiae/S. japonicus | (105 cell/mL)/(107 cell/mL) Ratio 1:100 | 3.49 ± 0.01 a | 5.40 ± 0.49 b | 0.36 ± 0.06 a | 13.75 ± 0.33 a | 0.28 ± 0.14 a,b | 0.27 ± 0.03 c | 12.12 ± 1.19 a | 1160 ± 72 b |
| S. cerevisiae/S. japonicus | (103 cell/mL)/(107 cell/mL) Ratio1:10,000 | 3.41 ± 0.13 a | 5.30 ± 0.35 b | 0.37 ± 0.08 a | 13.34 ± 0.27 a | 0.58 ± 0.32 a | 0.31 ± 0.02 c | 13.08 ± 1.39 a | 1427 ± 40 a |
| Yeasts | Inoculum Cell Concentration | Acetaldehyde (mg/L) | Ethyl Acetate (mg/L) | Ethyl lactate (mg/L) | Propanol (mg/L) | Isobutanol (mg/L) | Amylic Alcohol (mg/L) | Isoamylic Alcohol (mg/L) |
|---|---|---|---|---|---|---|---|---|
| S. cerevisiae | (107 cell/mL) | 31.61 ± 2.8 b | 30.74 ± 2.1 c | 8.95 ± 0.7 a | 36.63 ± 4.0 a | 69.55 ± 2.2 c,d | 12.33 ± 0.4 c | 113.21 ± 1.9 c |
| S. cerevisiae | (105 cell/mL) | 33.34 ± 2.3 b | 32.45 ± 1.0 c | 8.73 ± 0.2 a,b | 37.88 ± 3.9 a | 77.17 ± 2.5 c | 11.54 ± 0.5 c | 108.42 ± 2.8 c |
| S. cerevisiae | (103 cell/mL) | 27.37 ± 0.0 c | 34.34 ± 2.6 c | 8.37 ± 0.3 b | 36.85 ± 2.8 a | 72.59 ± 2.0 c,d | 11.74 ± 0.1 c | 105.32 ± 8.2 c |
| S. cerevisiae/S. japonicus | (107 cell/mL)/(107 cell/mL) (Ratio 1:1) | 49.40 ± 2.1 a | 156.60 ± 2.8 b | 8.30 ± 0.4 a,b | 28.10 ± 2.7 b | 159.60 ± 0.5 b | 57.30 ± 4.7 b | 348.80 ± 5.7 b |
| S. cerevisiae/S. japonicus | (105 cell/mL)/(107 cell/mL) (Ratio 1:100) | 32.90 ± 4.1 b | 213.70 ± 6.4 a | 7.48 ± 0.3 b,c | 18.90 ± 1.5 c | 202.80 ± 4.2 a | 59.10 ± 4.9 a | 395.70 ± 21.2 a |
| S. cerevisiae/S. japonicus | (103 cell/mL)/(107 cell/mL) (Ratio1:10,000) | 25.40 ± 3.7 c | 216.70 ± 8.5 a | 3.86 ± 0.2 c | 18.80 ± 1.5 c | 203.20 ± 8.5 a | 59.10 ± 1.4 a | 401.10 ± 24.4 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, C.; Lencioni, L.; Gobbi, M.; Mannazzu, I.; Ciani, M.; Domizio, P. Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking. Fermentation 2018, 4, 14. https://doi.org/10.3390/fermentation4010014
Romani C, Lencioni L, Gobbi M, Mannazzu I, Ciani M, Domizio P. Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking. Fermentation. 2018; 4(1):14. https://doi.org/10.3390/fermentation4010014
Chicago/Turabian StyleRomani, Cristina, Livio Lencioni, Mirko Gobbi, Ilaria Mannazzu, Maurizio Ciani, and Paola Domizio. 2018. "Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking" Fermentation 4, no. 1: 14. https://doi.org/10.3390/fermentation4010014
APA StyleRomani, C., Lencioni, L., Gobbi, M., Mannazzu, I., Ciani, M., & Domizio, P. (2018). Schizosaccharomyces japonicus: A Polysaccharide-Overproducing Yeast to Be Used in Winemaking. Fermentation, 4(1), 14. https://doi.org/10.3390/fermentation4010014