Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects
Abstract
:1. Introduction
2. Microalgae for Commercial Cultivation and Their Growth Conditions
2.1. Haematococcus pluvialis
2.2. Arthrospira (Spirulina) spp.
2.3. Dunaliella spp.
2.4. Chlorococcum sp.
2.5. Porphyridium spp.
2.6. Phaeodactylum tricornutum
2.7. Crypthecodinium cohnii
3. Outdoor and Indoor Cultivation of Microalgae
- (1)
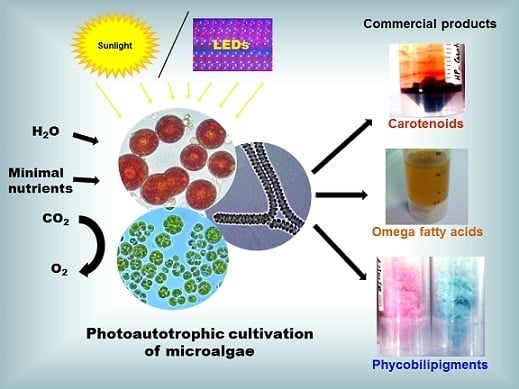
- Photoautotrophic cultivation: This is the most commonly used microalgae cultivation condition that uses lights, such as sunlight or artificial lights that supply photosynthetically active radiation (PAR, 400–700 nm) as an energy source, and inorganic carbon (mostly as CO2 gas in air and certain instances chemical CO2 as sodium bicarbonate) as the carbon source to carry out the photosynthesis for the first product glucose. Nowadays, the use of LED (light emitting diodes) lights [29,38] as source of energy for photoautotrophic cultivation is developing because of the fact of low energy consumption as well as the supplying of narrow range lights (e.g., red LED, 624–634 nm; green LED, 515–525 nm; blue LED; 460–465 nm) for the enhancement of specific biomolecule production. The biomass yield and especially lipid productivity were reported to increase by using 2% CO2 in air [67]. However, to reduce the cost and also to recycle industrial CO2, the microalgae cultivation facility should not be far away from the CO2 source.
- (2)
- Heterotrophic cultivation: The organisms capable of heterotrophic cultivation lack the photosynthetic machinery and hence cannot generate energy through inorganic compounds oxidation [68]. Heterotrophic cultivation requires organic carbon (glucose, fructose, sucrose, lactose, galactose, mannose, acetate, glycerol, etc.) as both the energy and carbon source. However, most microalgae prefer glucose as it can easily be assimilated and produce energy-rich compounds such as neutral lipids. Glucose-grown microalgae showed higher growth rates compared to those grown on acetate and fructose [68]. The yield of lutein was found to be increased with the increase in glucose concentration in C. protothecoides [69]. However, under heterotrophic cultivation H. pluvialis grew very slowly and accumulated only 0.5% astaxanthin of dry weight biomass [70]. Certain microalgae are not obligate photoautotrophs and in fact prefer using organic carbon under dark cycle of growth, which is considered as heterotrophic microalgae. Heterotrophic cultivation was reported to be associated with higher biomass production and lipid productivity in Chlorella protothecoides [71]. However, cultivating microalgae under this condition may be challenging as this suffers from contamination problems and hence, maintenance of sterile seed-culture is very important. The merit for this type of microalgae cultivation includes the avoidance of lights limitation as faced in the high-density microalgae cultures in large-scale photobioreactors [72].
- (3)
- Mixotrophic cultivation: This is an interesting capacity of certain microalgae that can perform photosynthesis using both organic carbon compounds and inorganic carbon (CO2) as a carbon source for their growth. These microalgae are facultative photoautotrophic or heterotrophic, or even both. Compared to photoautotrophic and heterotrophic cultivation, mixotrophic cultivation of microalgae for nutraceutical applications is rare except reports for astaxanthin production in H. pluvialis [30] and increased biomass in Arthrospira (Spirulina) [36]. Under mixotrophic conditions, both growth and astaxanthin production in H. pluvialis were found to be increased [73]. Therefore, mixotrophic cultivation is more prefered for enhanced production of biomass, lipid and carotenoids yield in microalgal species.
- (4)
- Photoheterotrophic cultivation: This is a typical type of cultivation where microalgae require light as an energy source while utilising organic compounds as the carbon source [74]. It appears that both mixotrophic and photoheterotrophic cultivations are the same but the subtle difference is that the mixotrophic cultures can use organic compounds as an energy source and photoheterotrophic cultures require light as the energy source.
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saha, S.K. Microalgae and cyanobacterial feedstocks for multiple commodities. In Feedstocks: Production Practices, Technologies and Environmental Impacts; Acquaye, A., Ed.; Nova Publishers: New York, NY, USA, 2014; pp. 73–107. [Google Scholar]
- Saha, S.K.; McHugh, E.; Murray, P.; Walsh, D.J. Microalgae as a source of nutraceuticals. In Phycotoxins: Chemistry and Biochemistry, 2nd, ed.; Botana, L.M., Alfonso, A., Eds.; John Wiley & Sons Ltd: Chichester, UK, 2015; pp. 255–292. [Google Scholar]
- Bondioli, P.; Della, B.L.; Rivolta, G.; Zittelli, G.C.; Bassi, N.; Rodolfi, L.; Casini, D.; Prussi, M.; Chiaramonti, D.; Tredici, M.R. Oil production by the marine microalgae Nannochloropsis sp. F&M-M24 and Tetraselmis suecica F&M-M33. Bioresour. Technol. 2012, 114, 567–572. [Google Scholar] [PubMed]
- Pruvost, J.; Van Vooren, G.; Le Gouic, B.; Couzinet-Mossion, A.; Legrand, J. Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour. Technol. 2011, 102, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2008, 54, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Comparison of heterotrophic and photoautotrophic induction on astaxanthin production by Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 2005, 68, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.; García-González, M.; Guerrero, M. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Yongmanitchai, W.; Ward, O.P. Growth of and omega-3 fatty acid production by Phaeodactylum tricornutum under different culture conditions. Appl. Environ. Microbiol. 1991, 57, 419–425. [Google Scholar] [PubMed]
- Winwood, R.J. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. Oilseeds Fats Crops Lipids 2013, 20, D604. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Mimouni, V.; Ulmann, L.; Tremblin, G. Combined effects of irradiance level and carbon source on fatty acid and lipid class composition in the microalga Pavlova lutheri commonly used in mariculture. J. Exp. Mar. Biol. Ecol. 2009, 369, 136–143. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P.K. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ben-Amotz, A.; Katz, A.; Avron, M. Accumulation of β-carotene in halotolerant algae: Purification and characterization of β-carotene rich globules from Dunaliella bardawil. J. Phycol. 1982, 18, 529–537. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. The carotenoid pigment zeaxanthin—A Review. Compr. Rev. Food Sci. Food Saf. 2008, 7, 29–49. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Complementary limiting factors of astaxanthin synthesis during photoautotrophic induction of Haematococcus pluvialis: C/N ratio and light intensity. Appl. Microbiol. Biotechnol. 2007, 74, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Auestad, N.; Scott, D.T.; Janowsky, J.S.; Jacobsen, C.; Carroll, R.E.; Montalto, M.B.; Halter, R.; Qiu, W.; Jacobs, J.R.; Connor, W.E.; et al. Visual, cognitive, and language assessments at 39 months: A follow-up study of children fed formulas containing long-chain polyunsaturated fatty acids to 1 year of age. Pediatrics 2003, 112, e177–e183. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease-but how and why? Prostaglandins Leukot. Essent. Fatty Acids 2008, 78, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Z.; Reungjitchachawali, M.; Siangdung, W.; Tanticharoen, M. Production and partial purification of γ-linolenic acid and some pigments from Spirulina platensis. J. Appl. Phycol. 1993, 5, 109–115. [Google Scholar] [CrossRef]
- Benedetti, S.; Benvenuti, F.; Pagliarani, S.; Francogli, S.; Scoglio, S.; Canestrari, F. Antioxidant properties of a novel phycocyanin extract from the blue-green alga Aphanizomenon flos-aquae. Life Sci. 2004, 75, 2353–2362. [Google Scholar] [CrossRef] [PubMed]
- Romay, C.P.; Gonazelz, R.; Ledon, N.; Remirez, D.; Rimbau, V. C-phycocyanin: A biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr. Protein Pept. Sci. 2003, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minkova, K.M.; Toshkova, R.A.; Gardeva, E.G.; Tchorbadjieva, M.I.; Ivanova, N.J.; Yossifova, L.S.; Gigova, L.G. Antitumor activity of B-phycoerythrin from Porphyridium cruentum. J. Pharm. Res. 2011, 4, 1480–1482. [Google Scholar]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Zhang, D.H. Production of astaxanthin by Haematococcus. In Chemicals from Microalgae; Cohen, Z., Ed.; Taylor & Francis: London, UK, 1999; pp. 173–195. [Google Scholar]
- Zhekisheva, M.; Boussiba, S.; Khozin-Goldberg, I.; Zarka, A.; Cohen, Z. Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J. Phycol. 2002, 38, 325–331. [Google Scholar] [CrossRef]
- Saha, S.K.; McHugh, E.; Hayes, J.; Moane, S.; Walsh, D.; Murray, P. Effect of various stress regulatory factors on biomass and lipid production in microalga Haematococcus pluvialis. Bioresour. Technol. 2013, 128, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Choi, S.P.; Hong, M.E.; Sim, S.J. Enhanced astaxanthin production from microalga, Haematococcus pluvialis by two-stage perfusion culture with step wise light irradiation. Bioprocess Biosyst. Eng. 2014, 37, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Damiani, M.C.; Popovich, C.A.; Constenla, D.; Leonardi, P.I. Lipid analysis in Haematococcus pluvialis to assess its potential use as a biodiesel feedstock. Bioresour. Technol. 2010, 101, 3801–3807. [Google Scholar] [CrossRef] [PubMed]
- John, R.P.; Anisha, G.S.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as substrates for fermentative biogas production in a combined biorefinery concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigam, P.S.; Singh, A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Mišurcová, L.; Škrovánková, S.; Samek, D.; Ambrožová, J.; Machů, L. Health benefits of algal polysaccharides in human nutrition. Adv. Food Nutr. Res. 2012, 66, 75–145. [Google Scholar] [PubMed]
- Chen, F.; Zhang, Y. High cell density mixotrophic culture of Spirulina platensis on glucose for phycocyanin production using a fed-batch system. Enzym. Microb. Technol. 1997, 20, 221–224. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Fu, C.C.; Liu, Y.C. Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem. Eng. J. 2007, 37, 21–25. [Google Scholar] [CrossRef]
- Jiménez, C.; Cossío, B.R.; Labella, D.; Niell, F.X. The feasibility of industrial production of Spirulina (Arthrospira) in Southern Spain. Aquaculture 2003, 217, 179–190. [Google Scholar] [CrossRef]
- De Bhowmick, G.; Subramanian, G.; Mishra, S.; Sen, R. Raceway pond cultivation of a marine microalga of Indian origin for biomass and lipid production: A case study. Algal Res. 2014, 6, 201–209. [Google Scholar] [CrossRef]
- Richmond, A.; Grobbelaar, J.U. Factors affecting the output rate of Spirulina platensis with reference to mass cultivation. Biomass 1986, 10, 253–264. [Google Scholar] [CrossRef]
- Tanticharoen, M.; Reungjitchachawali, M.; Boonag, B.; Vonktaveesuk, P.; Vonshak, A.; Cohen, Z. Optimization of gamma-linolenic acid (GLA) production in Spirulina platensis. J. Appl. Phycol. 1994, 6, 295–300. [Google Scholar] [CrossRef]
- Hadi, M.R.; Shariati, M.; Afsharzadeh, S. Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. Biotechnol. Bioproc. Eng. 2008, 13, 540–544. [Google Scholar] [CrossRef]
- Kaçka, A.; Dönmez, G. Isolation of Dunaliella spp. from a hypersaline lake and their ability to accumulate glycerol. Bioresour. Technol. 2008, 99, 8348–8352. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Paglia, G.; Magnúsdóttir, M.; Steinarsdóttir, E.A.; Gudmundsson, S.; Palsson, B.Ø.; Andrésson, Ó.S.; Brynjólfsson, S. Effects of abiotic stressors on lutein production in the green microalga Dunaliella salina. Microb. Cell Fact. 2014, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Jha, B. Antioxidant response of the microalga Dunaliella salina under salt stress. Bot. Mar. 2011, 54, 195–199. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol. 1983, 72, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Gómez, P.I.; González, M.A. The effect of temperature and irradiance on the growth and carotenogenic capacity of seven strains of Dunaliella salina (Chlorophyta) cultivated under laboratory conditions. Biol. Res. 2005, 38, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Preetha, K.; John, L.; Subin, C.S.; Vijayan, K.K. Phenotypic and genetic characterization of Dunaliella (Chlorophyta) from Indian salinas and their diversity. Aquat. Biosyst. 2012, 8, 27. Available online: http://www.aquaticbiosystems.org/content/8/1/27 (accessed on 13 June 2018). [CrossRef] [PubMed]
- Tafreshi, A.H.; Shariati, M. Dunaliella biotechnology: Methods and applications. J. Appl. Microbiol. 2009, 107, 14–35. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Chi, C.H. Process development and evaluation for algal glycerol production. Biotechnol. Bioeng. 1981, 23, 1267–1287. [Google Scholar] [CrossRef]
- Ben-Amotz, A. New mode of Dunaliella biotechnology: Two-phase growth for β-carotene production. J. Appl. Phycol. 1995, 7, 65–68. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F.; Liu, X.; Lic, X.-Z. Carotenoid composition in the green microalga Chlorococcum. Food Chem. 2002, 76, 319–325. [Google Scholar] [CrossRef]
- Zhang, D.H.; Lee, Y.K. Ketocarotenoid production by a mutant of Chlorococcum sp. in an outdoor tubular photobioreactor. Biotechnol. Lett. 1999, 21, 7–10. [Google Scholar] [CrossRef]
- Beevi, U.S.; Sukumaran, R.K. Cultivation of the fresh water microalga Chlorococcum sp. RAP13 in sea water for producing oil suitable for biodiesel. J. Appl. Phycol. 2015, 27, 141–147. [Google Scholar] [CrossRef]
- Velea, S.; Ilie, L.; Filipescu, L. Optimization of Porphyridium purpureum culture growth using two variables experimental design: Light and sodium bicarbonate. UPB Sci. Bull. 2011, 73, 81–94. [Google Scholar]
- Fuentes, R.M.; Fernandez, A.G.; Prerez, S.J.; Guerrero, G.J. Biomass, nutrient profiles of the microalga Porphyridium purpureum. Food Chem. 2000, 70, 345–353. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Asgharpour, M.; Rodgers, B.; Hestekin, J.A. Eicosapentaenoic acid from Porphyridium cruentum: Increasing growth and productivity of microalgae for pharmaceutical products. Energies 2015, 8, 10487–10503. [Google Scholar] [CrossRef]
- Fuentes, M.R.; Sánchez, J.G.; Sevilla, J.F.; Fernández, F.A.; Pérez, J.S.; Grima, E.M. Outdoor continuous culture of Porphyridium cruentum in a tubular photobioreactor: Quantitative analysis of the daily cyclic variation of culture parameters. J. Biotechnol. 1999, 70, 271–288. [Google Scholar] [CrossRef]
- Oh, S.O.; Han, J.G.; Kim, Y.; Ha, J.H.; Kim, S.S.; Jeong, M.H.; Jeong, H.S.; Kim, N.Y.; Cho, J.S.; Yoon, W.B.; et al. Lipid production in Porphyridium cruentum grown under different culture conditions. J. Biosci. Bioeng. 2009, 108, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Miron, A.; Ceron Garcia, M.C.; Contreras Gomez, A.; Garcia Camacho, F.; Molina Grima, E.; Chisti, Y. Shear stress tolerance and biochemical characterization of Phaeodactylum tricornutum in quasi steady-state continuous culture in outdoor photobioreactors. Biochem. Eng. J. 2003, 16, 287–297. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Han, X.; Yu, Z. A rare Phaeodactylum tricornutum cruciform morphotype: Culture conditions, transformation and unique fatty acid characteristics. PLoS ONE 2014, 9, e93922. [Google Scholar] [CrossRef] [PubMed]
- Meiser, A.; Schmid-Staiger, U.; Trosch, W. Optimization of eicosapentaenoic acid production by Phaeodactylum tricornutum in the flat panel airlift (FPA) reactor. J. Appl. Phycol. 2004, 16, 215–225. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; San Martin, S.; Agurto, C.; Santos, M.A.; Freitas, M.A.V.; Mata, T.M.; Martins, A.A.; Caetano, N.S. Potential of Phaeodactylum tricornutum for biodiesel production under natural conditions in Chile. Energies 2018, 11, 54. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Chen, C.H.; Kuan, T.C.; Ong, S.C.; Lin, C.S. Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour. Technol. 2008, 99, 3389–3396. [Google Scholar] [CrossRef] [PubMed]
- Minhas, A.K.; Hodgson, P.; Barrow, C.J.; Adholeya, A. A review on the assessment of stress conditions for simultaneous production of microalgal lipids and carotenoids. Front. Microbiol. 2016, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-M.; Zhang, X.-W.; Chen, F. Heterotrophic production of biomass and lutein by Chlorella protothecoides on various nitrogen sources. Enzym. Microb. Technol. 2000, 27, 312–318. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kakizono, T.; Yamaguchi, K.; Nishio, N.; Nagai, S. Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic condition. J. Ferment. Bioeng. 1992, 74, 17–20. [Google Scholar] [CrossRef]
- Xu, H.; Miao, X.L.; Wu, Q.Y. High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J. Biotechnol. 2006, 126, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.H.; Chen, F.; Wei, D.; Zhang, X.W.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Wang, S.B.; Chen, F.; Sommerfeld, M.; Hu, Q. Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 2004, 220, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K.; Marquez-Rocha, F.J. Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 2004, 3, 21–34. [Google Scholar]
- Uchida, M.; Miyoshi, T. Algal fermentation-the seed for a new fermentation industry of foods and related products. JARQ 2013, 47, 53–63. Available online: http://www.jircas.affrc.go.jp (accessed on 13 June 2018). [CrossRef]
- Uchida, M. Analysis and collection of data on algal fiber and sugar contents for fermentative utilization of seaweed biomass. SEN’I GAKKAISHI 2011, 67, 181–186. [Google Scholar] [CrossRef]
- Petr, K.; Olga, K.; Frantisek, K.; Irena, B.; Gita, P.; Jitka, J.; Tomáš, B.; Katerina, B. Selective bioaccumulation of rubidium by microalgae from industrial wastewater containing rubidium and lithium. J. Appl. Phycol. 2018, 30, 461–467. [Google Scholar]
- Chen, C.Y.; Yeh, K.L.; Aisyah, R.; Lee, D.J.; Chang, J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review. Bioresour. Technol. 2011, 102, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Posten, C. Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Narala, R.R.; Garg, S.; Sharma, K.K.; Thomas-Hall, S.R.; Deme, M.; Li, Y.; Schenk, P.M. Comparison of microalgae cultivation in photobioreactor, open raceway pond, and a two-stage hybrid system. Front. Energy Res. 2016, 4, 29. [Google Scholar] [CrossRef]
- Tylor, J.; Johnson, T.J.; Katuwal, S.; Anderson, G.A.; Gu, L.; Zhou, R.; Gibbons, W.R. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 2018. [Google Scholar] [CrossRef]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals from algae and cyanobacteria. In Algal Green Chemistry, Recent Progress in Biotechnology; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Saint Louis, MO, USA, 2017; pp. 65–89. [Google Scholar]




| Microalgae Species | Cultivation History | Growth Type | Cultivation Conditions | Applications |
|---|---|---|---|---|
| Chlorella vulgaris | 1951 | Photoautotrophic | Open raceway pond, tubular PBR, flat-plate photobioreactor | Whole biomass for human nutrition as tablets, powders, nectar noodles; cosmetics; aquafeed |
| Crypthecodinium cohnii | 1999 | Heterotrophic | Large stainless steel Fermentor | DHASCO™ oil for the infant formula as DHA source |
| Dunaliella salina | 1980 | Photoautotrophic (two phase cultivation) | Unstirred open pond, lagoons, paddle wheel stirred raceway ponds, tubular photobioreactors | Carotenoid β-carotene for food and in cosmetics, human nutrition as powder, animal feed, source for proteins and glycerol |
| Haematococcus pluvialis | 2000 | Photoautotrophic (two phase cultivation), Mixotrophic | Open raceway pond, tubular enclosed outdoor PBR, bubble column and airlift photobioreactors, large plastic bags | Carotenoid astaxanthin, aquafeed, poultry feed, animal feed, human nutrition, cosmetics, pharmaceuticals, food-colourant, food-supplement |
| Nannochloropsis sp. | 1997 | Photoautotrophic, Mixotrophic | Raceway pond, Helical-tubular photobioreactor | EPA oil for human nutrition, aquaculture |
| Odontella aurita | 1996 | Photoautotrophic, heterotrophic or mixotrophic | outdoor open ponds, Pilot Tanks, cylindrical glass columns and flat-plate photobioreactors | Human nutrition, baby food as EPA and DHA source, cosmetics |
| Phaeodactylum tricornutum | 1996 | Photoautotrophic | Open pond, circular tanks, outdoor pilot-scale bubble column photobioreactor, large 400 L polyethylene bags supported by frames, air-lift photobioreactor | Aqauculture feed, EPA oil as health supplement |
| Porphyridium cruentum | 1970 | Photoautotrophic | Tubular PBR | Pink phycoerythrin pigment, sulfated polysaccharide, cosmetics |
| Schizochytrium sp. | 1999 | Heterotrophic | Large stainless steel Fermentor | Life’s Omega™ oil as source for DHA and EPA |
| Arthrospira (Spirulina) platensis | 1970 | Photoautotrophic | Open raceway pond, tanks, earthen pots, basins, natural lakes | Whole biomass for human nutrition as tablets, capsules, powders; blue phycocyanin as colourant in food and in cosmetics; source for g-linolenic acid (GLA), vitamins and minerals |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, S.K.; Murray, P. Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects. Fermentation 2018, 4, 46. https://doi.org/10.3390/fermentation4020046
Saha SK, Murray P. Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects. Fermentation. 2018; 4(2):46. https://doi.org/10.3390/fermentation4020046
Chicago/Turabian StyleSaha, Sushanta Kumar, and Patrick Murray. 2018. "Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects" Fermentation 4, no. 2: 46. https://doi.org/10.3390/fermentation4020046
APA StyleSaha, S. K., & Murray, P. (2018). Exploitation of Microalgae Species for Nutraceutical Purposes: Cultivation Aspects. Fermentation, 4(2), 46. https://doi.org/10.3390/fermentation4020046