Fermentation as an Alternative Process for the Development of Bioinsecticides
Abstract
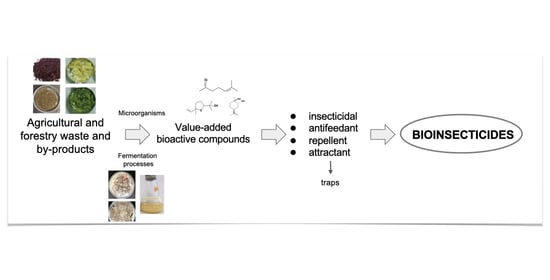
:1. Introduction
2. Fermentation Products—Insect Interaction
3. Fermentation of Plant-Based Materials as Source of Biopesticide Compounds
4. Fermentation of Pure Secondary Metabolites
4.1. Terpenes
4.2. Phenols
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, A.; Bose, S.; Wang, J.H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of vegetable waste into value added products: (A) the upgrading concept; (B) practical implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Kagliwal, L.D.; Singhal, R.S. Biotransformation of Polyphenols for Improved Bioavailability and Processing Stability. In Advances in Food and Nutrition Research; Taylor, S., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2013; Volume 69, pp. 183–217. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Papaspyridi, L.M.; Aligiannis, N.; Topakas, E.; Christakopoulos, P.; Skaltsounis, A.L.; Fokialakis, N. Submerged fermentation of the edible mushroom Pleurotus ostreatus in a batch stirred tank bioreactor as a promising alternative for the effective production of bioactive metabolites. Molecules 2012, 17, 2714–2724. [Google Scholar] [CrossRef]
- Zhou, J.; Du, G.; Chen, J. Novel fermentation processes for manufacturing plant natural products. Curr. Opin. Biotechnol. 2014, 25, 17–23. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef]
- Rodríguez, P.; Cerda, A.; Font, X.; Sánchez, A.; Artola, A. Valorisation of biowaste digestate through solid state fermentation to produce biopesticides from Bacillus thuringiensis. Waste Manag. 2019, 93, 63–71. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Mitchell, D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochem. 2000, 35, 1153–1169. [Google Scholar] [CrossRef]
- Postemsky, P.D.; Bidegain, M.A.; Lluberas, G.; Lopretti, M.I.; Bonifacino, S.; Inés Landache, M.; Zygadlo, J.A.; Fernández-Lahore, M.; Omarini, A.B. Biorefining via solid-state fermentation of rice and sunflower by-products employing novel monosporic strains from Pleurotus sapidus. Bioresour. Technol. 2019, 289, 121692. [Google Scholar] [CrossRef]
- Omarini, A.; Lechner, B.E.; Albertó, E. Polyporus tenuiculus: A new naturally occurring mushroom that can be industrially cultivated on agricultural waste. J. Ind. Microbiol. Biotechnol. 2009, 36, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Omarini, A.B.; Labuckas, D.; Zunino, M.P.; Pizzolitto, R.; Fernández-Lahore, M.; Barrionuevo, D.; Zygadlo, J.A. Upgrading the nutritional value of rice bran by Solid-State fermentation with Pleurotus sapidus. Fermentation 2019, 5, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Omarini, A.; Dambolena, J.S.; Lucini, E.; Jaramillo Mejía, S.; Albertó, E.; Zygadlo, J.A. Biotransformation of 1,8-cineole by solid-state fermentation of Eucalyptus waste from the essential oil industry using Pleurotus ostreatus and Favolus tenuiculus. Folia Microbiol. Praha. 2016, 61, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; Martínez-Burgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst. Microbiol. Biomanufacturing 2020. [Google Scholar] [CrossRef]
- De La Cruz Quiroz, R.; Roussos, S.; Hernández, D.; Rodríguez, R.; Castillo, F.; Aguilar, C.N. Challenges and opportunities of the bio-pesticides production by solid-state fermentation: Filamentous fungi as a model. Crit. Rev. Biotechnol. 2015, 35, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Chio, E.H. Insecticides from Fermentation Secondary Metabolites. Formos. Entomol. 2007, 27, 97–106. [Google Scholar]
- Matsuda, K. Okaramines and other plant fungal products as new insecticide leads. Curr. Opin. Insect Sci. 2018, 30, 67–72. [Google Scholar] [CrossRef]
- Marrone, P.G. The Market and Potential for Molecular POC Diagnostics. In Biopesticides: State of the Art and Future Opportunities; Gross, A.D., Coats, J.R., Duke, S.O., Seiber, J.N., Eds.; ACS Publications: Washington DC, USA, 2014; Volume 9, pp. 245–258. [Google Scholar]
- Yoshimoto, J.; Kakutani, T.; Nishida, T. Influence of resource abundance on the structure of the insect community attracted to fermented tree sap. Ecol. Res. 2005, 20, 405–414. [Google Scholar] [CrossRef]
- Cha, D.H.; Adams, T.; Werle, C.T.; Sampson, B.J.; Adamczyk, J.J.; Rogg, H.; Landolt, P.J. A four-component synthetic attractant for Drosophila suzukii (Diptera: Drosophilidae) isolated from fermented bait headspace. Pest Manag. Sci. 2014, 70, 324–331. [Google Scholar] [CrossRef]
- Kim, G.; Huang, J.H.; McMullen, J.G.; Newell, P.D.; Douglas, A.E. Physiological responses of insects to microbial fermentation products: Insights from the interactions between Drosophila and acetic acid. J. Insect Physiol. 2018, 106, 13–19. [Google Scholar] [CrossRef]
- Christiaens, J.F.; Franco, L.M.; Cools, T.L.; de Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef]
- Ômura, H.; Honda, K.; Asaoka, K.; Inoue, T.A. Divergent behavioral and electrophysiological taste responses in the mid-legs of adult butterflies, Vanessa indica and Argyreus hyperbius. J. Insect Physiol. 2011, 57, 118–126. [Google Scholar] [CrossRef]
- Brown, R.L.; El-Sayed, A.M.; Unelius, C.R.; Suckling, D.M. Attraction of the invasive social wasp, Vespula vulgaris, by volatiles from fermented brown sugar. Entomol. Exp. Appl. 2014, 151, 182–190. [Google Scholar] [CrossRef]
- Nigam, P.S. Production of Bioactive Secondary Metabolites. In Biotechnology for Agro-Industrial Residues Utilisation; Nigam, P.S., Pandey, A., Eds.; Springer Nature: Dordrecht, Switzerland, 2009; pp. 129–145. [Google Scholar]
- Olukunle, O.F.; Sanusi, A.I. Microbial and Physicochemical Properties of Fermented African Locust Bean (Parkia biglobosa) Effluent and its Biocidal Potential on some Selected Insects. Int. J. Sci. 2018, 7, 49–56. [Google Scholar] [CrossRef]
- Nzanza, B.; Mashela, P.W. Control of whiteflies and aphids in tomato (Solanum lycopersicum L.) by fermented plant extracts of neem leaf and wild garlic. Afr. J. Biotechnol. 2012, 11, 16077–16082. [Google Scholar] [CrossRef] [Green Version]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manage. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- de Ramos, A.S.; Ribeiro, J.B.; Teixeira, B.G.; Ferreira, J.L.P.; de Silva, J.R.A.; do Ferreira, A.A.; de Souza, R.O.M.A.; Amaral, A.C.F. Hydroxylation of 1,8-cineole by Mucor ramannianus and Aspergillus niger. Braz. J. Microbiol. 2015, 46, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Nájera, V.C.; Lugo-Cervantes, E.; Amaya-Delgado, L.; Madrigal-Pulido, J.A.; Rueda-Puente, E.O.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Biotransformation of hesperidin from lime peel (Citrus limetta Risso) in solid fermentation by Aspergillus saitoi. CYTA J. Food 2018, 16, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Su, Q.; Zhou, Z.; Zhang, J.; Shi, C.; Zhang, G.; Jin, Z.; Wang, W.; Li, C. Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae). Entomol. Res. 2018, 48, 18–26. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Kharat, P.; Sarkar, P.; Mouliganesh, S.; Tiwary, V.; Priya, V.B.R.; Sree, N.Y.; Annapoorna, H.V.; Saikia, D.K.; Mahanta, K.; Thirumurugan, K. Ellagic acid prolongs the lifespan of Drosophila melanogaster. GeroScience 2020, 42, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Larios-Cruz, R.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Prado-Barragán, A.; Wong-Paz, J.E.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Utilization of Citrus Waste Biomass for Antioxidant Production by Solid-State Fermentation. In Waste to Wealth, Energy, Environment, and Sustainability; Singhania, R., Agarwal, R., Kumar, R., Sukumaran, R., Eds.; Springer: Singapore, 2018; pp. 83–96. [Google Scholar]
- Singh, B.; Kaur, T.; Kaur, S.; Manhas, R.K.; Kaur, A. Insecticidal potential of an endophytic Cladosporium velox against Spodoptera litura mediated through inhibition of alpha glycosidases. Pestic. Biochem. Physiol. 2016, 131, 46–52. [Google Scholar] [CrossRef]
- Liu, C.; Hou, W.; Li, S.; Tsao, R. Extraction and isolation of acetylcholinesterase inhibitors from Citrus limon peel using an in vitro method. J. Sep. Sci. 2020, 43, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.M.; Fernandes, J.B.; Carlos, R.M.; Fernandes, M.N. Biochemical and genotoxic biomarkers and cell cycle assessment in the zebrafish liver (ZF-L) cell line exposed to the novel metal-insecticide magnesium-hespiridin complex. Chemosphere 2020, 250. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zhu, Y.W.; Jiang, Y.W.; Li, H.K.; Liu, Z.M.; Wang, W.; Shan, C.H.; Fu, Y.J. Improvement of flavonoid aglycone and biological activity of mulberry leaves by solid-state fermentation. Ind. Crops Prod. 2020, 148, 112287. [Google Scholar] [CrossRef]
- Harwoko, H.; Hartmann, R.; Daletos, G.; Ancheeva, E.; Frank, M.; Liu, Z.; Proksch, P. Biotransformation of Host Plant Flavonoids by the Fungal Endophyte Epicoccum nigrum. ChemistrySelect 2019, 4, 13054–13057. [Google Scholar] [CrossRef] [Green Version]
- Ghani, N.A.; Ismail, N.H.; Asakawa, Y. Constituents of fermented male flowers of Alnus sieboldiana (Betulaceae). Nat. Prod. Commun. 2017, 12, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Pei, J.; Chen, A.; Dong, P.; Shi, X.; Zhao, L.; Cao, F.; Tang, F. Modulating heterologous pathways and optimizing fermentation conditions for biosynthesis of kaempferol and astragalin from naringenin in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2019, 46, 171–186. [Google Scholar] [CrossRef]
- Sharma, R.; Sohal, S.K. Oviposition response of melon fruit fly, Bactrocera cucurbitae (Coquillett) to different phenolic compounds. J. Biopestic. 2016, 9, 46–51. [Google Scholar]
- Ji, Y.; Li, B.; Qiao, M.; Li, J.; Xu, H.; Zhang, L.; Zhang, X. Advances on the in vivo and in vitro glycosylations of flavonoids. Appl. Microbiol. Biotechnol. 2020, 104, 6587–6600. [Google Scholar] [CrossRef] [PubMed]
- Queiroz Santos, V.A.; Nascimento, C.G.; Schimidt, C.A.P.; Mantovani, D.; Dekker, R.F.H.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Dou, F.; Wang, Z.; Li, G.; Dun, B. Microbial transformation of flavonoids by Isaria fumosorosea ACCC 37814. Molecules 2019, 24, 1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makenzi, A.M.; Manguro, L.A.; Owuor, P.O.; Opiyo, S.A. Flavonol glycosides with insecticidal activity from methanol extract of Annona mucosa Jacq. leaves. Trends Phytochem. Res. 2019, 3, 287–296. [Google Scholar] [CrossRef]
- Aboshi, T.; Ishiguri, S.; Shiono, Y.; Murayama, T. Flavonoid glycosides in Malabar spinach Basella alba inhibit the growth of Spodoptera litura larvae. Biosci. Biotechnol. Biochem. 2018, 82, 9–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.G.; Yang, S.M.; Kim, S.Y.; Cha, M.N.; Ahn, J.H. Biosynthesis and production of glycosylated flavonoids in Escherichia coli: Current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 2979–2988. [Google Scholar] [CrossRef]
- Pandey, R.P.; Parajuli, P.; Chu, L.L.; Kim, S.Y.; Sohng, J.K. Biosynthesis of a novel fisetin glycoside from engineered Escherichia coli. J. Ind. Eng. Chem. 2016, 43, 13–19. [Google Scholar] [CrossRef]
- Muñoz, R.; de las Rivas, B.; López de Felipe, F.; Reverón, I.; Santamaría, L.; Esteban-Torres, M.; Curiel, J.A.; Rodríguez, H.; Landete, J.M. Biotransformation of Phenolics by Lactobacillus plantarum in Fermented Foods. In Fermented Foods in Health and Disease Prevention; Frías, J., Villaluenga, C.M., Peñas, E., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 63–83. [Google Scholar]
- Chauhan, N.S.; Sohal, S.K. Disruptive effect of pyrogallol on development of Spodoptera litura (Fab.) larvae. J. Biopestic. 2018, 11, 7–13. [Google Scholar]
- Abdulla, S.W. Identification of Gallic acid and Hydroquinone in the Propolis and their effects on ovaries of Khapra beetle Trogoderma granarium Everts Coleoptera: Dermestidae 2. Polytechnic 2016, 6, 281–286. [Google Scholar]
- Ahmed, A.; Abou-Taleb, K. Implementation of Different Fermentation Techniques for Induction of Tannase and Gallic Acid Using Agro-residues Substrates. Egypt. J. Microbiol. 2019, 54, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Abd Razak, D.L.; Abd Rashid, N.Y.; Jamaluddin, A.; Sharifudin, S.A.; Abd Kahar, A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sun, X.Q.; Yan, S.Y.; Pan, W.J.; Zhang, M.X.; Cai, Q.N. Interaction of Ferulic Acid with Glutathione S-Transferase and Carboxylesterase Genes in the Brown Planthopper, Nilaparvata lugens. J. Chem. Ecol. 2017, 43, 693–702. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal properties of phenols on Culex quinquefasciatus Say and Musca domestica L. Parasitol. Res. 2011, 109, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.M.; Feng, M.G. Aphidicidal activity of a novel botanical insecticide made by alkalization of bamboo tar. Crop Prot. 2016, 87, 85–89. [Google Scholar] [CrossRef]
- Hagner, M.; Tiilikkala, K.; Lindqvist, I.; Niemelä, K.; Wikberg, H.; Källi, A.; Rasa, K. Performance of Liquids from Slow Pyrolysis and Hydrothermal Carbonization in Plant Protection. Waste Biomass Valorization 2020, 11, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R. Antifeedant and larvicidal effects of some phenolic components of essential oils lasp lines of introduction against Spodoptera littoralis (boisd.). J. Essent. Oil-Bear. Plants 2011, 14, 266–273. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, R.; Zhang, Y.; Yang, Y.; Sun, X.; Zhang, Q.; Yang, N. Biotransformation of phenolics and metabolites and the change in antioxidant activity in kiwifruit induced by Lactobacillus plantarum fermentation. J. Sci. Food Agric. 2020, 100, 3283–3290. [Google Scholar] [CrossRef]
- Maazoun, A.M.; Hlel, T.B.; Hamdi, S.H.; Belhadj, F.; Jemâa, J.M.B.; Marzouki, M.N. Screening for insecticidal potential and acetylcholinesterase activity inhibition of Urginea maritima bulbs extract for the control of Sitophilus oryzae (L.). J. Asia. Pac. Entomol. 2017, 20, 752–760. [Google Scholar] [CrossRef]
- Fraga, B.M.; González-Coloma, A.; Alegre-Gómez, S.; López-Rodríguez, M.; Amador, L.J.; Díaz, C.E. Bioactive constituents from transformed root cultures of Nepeta teydea. Phytochemistry 2017, 133, 59–68. [Google Scholar] [CrossRef]
- Wu, C.; Li, T.; Qi, J.; Jiang, T.; Xu, H.; Lei, H. Effects of lactic acid fermentation-based biotransformation on phenolic profiles, antioxidant capacity and flavor volatiles of apple juice. LWT 2020, 122, 109064. [Google Scholar] [CrossRef]
- Stuhl, C.J. Does Prior Feeding Behavior by Previous Generations of the Maize Weevil (Coleoptera: Curculionidae) Determine Future Descendants Feeding Preference and Ovipositional Suitability? Fla. Entomol. Soc. 2019, 102, 366–372. [Google Scholar] [CrossRef] [Green Version]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Brito, V.D.; Achimón, F.; Dambolena, J.S.; Pizzolitto, R.P.; Zygadlo, J.A. Trans-2-hexen-1-ol as a tool for the control of Fusarium verticillioides in stored maize grains. J. Stored Prod. Res. 2019, 82. [Google Scholar] [CrossRef]
- Kamauchi, H.; Kon, T.; Kinoshita, K.; Takahashi, K.; Koyama, K. Three new terpenoids, sterebins O, P1, and P2, isolated from Stevia rebaudiana fermented by Saccharomyces cerevisiae. Tetrahedron Lett. 2014, 55, 7203–7205. [Google Scholar] [CrossRef]
- Dong, J.W.; Cai, L.; Li, X.J.; Shi, Y.X.; Wang, J.P.; Mei, R.F.; Ding, Z.T. A new menthane-type monoterpenoid from fermented Illigera aromatica with Clonostachys rogersoniana 828H2. J. Asian Nat. Prod. Res. 2019, 21, 673–678. [Google Scholar] [CrossRef]
- Kettering, M.; Valdivia, C.; Sterner, O.; Anke, H.; Thines, E. Heptemerones A-G, seven novel diterpenoids from Coprinus heptemerus: Producing organism, fermentation, isolation and biological activities. J. Antibiot. Tokyo 2005, 58, 390–396. [Google Scholar] [CrossRef]
- Çorbacı, C. Biotransformation of terpene and terpenoid derivatives by Aspergillus niger NRRL 326. Biol. Bratisl. 2020, 75, 1473–1481. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Mishra, S. Plant Monoterpenoids (Prospective Pesticides). In Ecofriendly Pest Management for Food Security; Academic Press: Cambridge, MA, USA, 2016; pp. 507–524. [Google Scholar]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kalscheur, N.A.; Kemp, K.E. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 2009, 102, 1567–1579. [Google Scholar] [CrossRef]
- Duetz, W.A.; Bouwmeester, H.; Van Beilen, J.B.; Witholt, B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl. Microbiol. Biotechnol. 2003, 61, 269–277. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef] [Green Version]
- Larios-Cruz, R.; Londoño-Hernández, L.; Gómez-García, R.; García-Galindo, I.; Sepulveda, L.; Rodríguez-Herrera, R.; Aguilar, C.N. Extraction of Bioactive Molecules through Fermentation and Enzymatic Assisted Technologies. In High Value Fermentation Products; Saran, S., Babu, V., Chaubey, A., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2019; Volume 1, pp. 27–59. [Google Scholar]
- Emara, T.E. Effect of 6-methyl-5-hepten-2-one on acetylcholinesterase activity, growth and development of Spodoptera litoralis. Egypt. J. Biol. 2004, 6, 136–146. [Google Scholar] [CrossRef]
- Germinara, G.S.; Ganassi, S.; Pistillo, M.O.; Di Domenico, C.; De Cristofaro, A.; Di Palma, A.M. Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS ONE 2017, 12, e0190454. [Google Scholar] [CrossRef] [Green Version]
- Baydar, H.; Baydar, N.G. The effects of harvest date, fermentation duration and Tween 20 treatment on essential oil content and composition of industrial oil rose (Rosa damascena Mill.). Ind. Crops Prod. 2005, 21, 251–255. [Google Scholar] [CrossRef]
- Steyer, D.; Erny, C.; Claudel, P.; Riveill, G.; Karst, F.; Legras, J.L. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013, 33, 228–234. [Google Scholar] [CrossRef]
- Soares Rodrigues, G.C.; Dos Santos Maia, M.; Muratov, E.N.; Scotti, L.; Scotti, M.T. Quantitative Structure-Activity Relationship Modeling and Docking of Monoterpenes with Insecticidal Activity against Reticulitermes chinensis Snyder and Drosophila melanogaster. J. Agric. Food Chem. 2020, 68, 4687–4698. [Google Scholar] [CrossRef]
- Sales, A.; Afonso, L.F.; Americo, J.A.; de Freitas Rebelo, M.; Pastore, G.M.; Bicas, J.L. Monoterpene biotransformation by Colletotrichum species. Biotechnol. Lett. 2018, 40, 561–567. [Google Scholar] [CrossRef]
- Baser, H.C.; Buchbauer, G. Handbook of Essential Oils. Science, Technology and Applications; CRC Press: Boca Raton, FL, USA, 2010; Volume 72, pp. 1–949. [Google Scholar]
- Janocha, S.; Schmitz, D.; Bernhardt, R. Terpene hydroxylation with microbial cytochrome p450 monooxygenases. Adv. Biochem. Eng. Biotechnol. 2015, 148, 215–250. [Google Scholar] [CrossRef]
- Coats, J.R.; Karr, L.L.; Drewes, C.D. Toxicity and Neurotoxic Effects of Monoterpenoids. In Insects and Earthworms; Hedin, P., Ed.; ACS Symposium Series: Washington, DC, USA, 1991; pp. 305–316. [Google Scholar]
- Kordali, Ş.; Usanmaz, A.; Bayrak, N.; Çakır, A. Fumigation of volatile monoterpenes and aromatic compounds against adults of Sitophilus granarius (L.) (coleoptera: Curculionidae). Rec. Nat. Prod. 2017, 11, 362–373. [Google Scholar]
- Cheng, B.Q.; Wei, L.J.; Lv, Y.B.; Chen, J.; Hua, Q. Elevating Limonene Production in Oleaginous Yeast Yarrowia lipolytica via Genetic Engineering of Limonene Biosynthesis Pathway and Optimization of Medium Composition. Biotechnol. Bioprocess Eng. 2019, 24, 500–506. [Google Scholar] [CrossRef]
- Mikami, Y. Microbial conversion of terpenoids. Biotechnol. Genet. Eng. Rev. 1988, 6, 271–320. [Google Scholar] [CrossRef]
- Nishimura, H.; Noma, Y.; Mizutan, J. Eucalyptus as biomass. Novel compounds from microbial conversion of 1,8-cineole. Agric. Biol. Chem. 1982, 46, 2601–2604. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural Products for Controlling Insects of Importance to Human Health—A Structure-Activity Relationship Study. Psyche Lond. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Mark, R.; Lyu, X.; Lee, J.J.L.; Parra-Saldívar, R.; Chen, W.N. Sustainable production of natural phenolics for functional food applications. J. Funct. Foods 2019, 57, 233–254. [Google Scholar] [CrossRef]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Huerta-Ochoa, S.; Castillo-Araiza, C.O.; Guerrero, A.R.; Prado-Barragán, A. Whole-Cell Bioconversion of Citrus Flavonoids to Enhance Their Biological Properties. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier B.V.: New York, NY, USA, 2018; Volume 61, pp. 335–367. [Google Scholar]
- Cao, H.; Chen, X.; Jassbi, A.R.; Xiao, J. Microbial biotransformation of bioactive flavonoids. Biotechnol. Adv. 2015, 33, 214–223. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2014, 33, 1063–1090. [Google Scholar] [CrossRef]
- Parshikov, I.A.; Sutherland, J.B. Biotransformation of Steroids and Flavonoids by Cultures of Aspergillus niger. Appl. Biochem. Biotechnol. 2015, 176, 903–923. [Google Scholar] [CrossRef]
- Begum, N.A.; Roy, N.; Laskar, R.A.; Roy, K. Mosquito larvicidal studies of some chalcone analogues and their derived products: Structure-activity relationship analysis. Med. Chem. Res. 2011, 20, 184–191. [Google Scholar] [CrossRef]
- Morimoto, M.; Komai, K. Insect antifeedant activity of natural products and the structure-activity relationship of their derivatives. In Natural Products for Pest Management; ACS Publishing: Washington, DC, USA, 2006; Volume 927, pp. 182–193. ISBN 0841239339. [Google Scholar]
- Romanelli, G.P.; Virla, E.G.; Duchowicz, P.R.; Gaddi, A.L.; Ruiz, D.M.; Bennardi, D.O.; Del Valle Ortiz, E.; Autino, J.C. Sustainable synthesis of flavonoid derivatives, QSAR study and insecticidal activity against the fall armyworm, Spodoptera frugiperda (Lep.: Noctuidae). J. Agric. Food Chem. 2010, 58, 6290–6295. [Google Scholar] [CrossRef]
- Duchowicz, P.R.; Goodarzi, M.; Ocsachoque, M.A.; Romanelli, G.P.; del Ortiz, E.V.; Autino, J.C.; Bennardi, D.O.; Ruiz, D.M.; Castro, E.A. QSAR analysis on Spodoptera litura antifeedant activities for flavone derivatives. Sci. Total Environ. 2009, 408, 277–285. [Google Scholar] [CrossRef]




| Fermented Substrate(s) | Microorganism | Product(s) | Reference |
|---|---|---|---|
| Eucalyptus leaves and 1,8-cineole | Pleurotus ostreatus Favolus tenuiculus | 1,3,3-trimethyl-2-oxabicyclo [2.2.2] octan-6-ol, and 1,3,3- trimethyl-2-oxabicyclo [2.2.2] octan-6-one | [14] |
| 1,8-cineole | Mucor ramannianus Aspergillus niger | 1,3,3-trimethyl-2-oxabicyclo [2.2.2] octan-6-ol, and 1,3,3- trimethyl-2-oxabicyclo [2.2.2] octan-6-one | [31] |
| Lime peel (hesperidin and naringin) | Aspergillus saitoi | 8-hydroxyhesperetin, 6-hydroxynaringenin, 8-hydroxynaringenin, eriodictyol, hesperetin, and naringenin | [32] |
| Orange peel (polyphenols) | Aspergillus fumigatus | Ellagic acid | [34] |
| Lemon, orange, grapefruit, and tangerine peels | Fusarium oxysporum Penicillium purpurogenu Trichoderma harzianum A. niger | Chlorogenic acid, didymin, apigenin 7-O-apiosyl-glucoside, pinoresinol, medioresinol, naringin, and hesperidin | [36] |
| Morus alba leaves | Monascus anka | Quercetin and kaempferol | [40] |
| Green lentils | Epicoccum nigrum | Kaempferol and kaempferol O-diglycosides | [41] |
| Alnus sieboldiana male flowers | Penicillium spp. | Kaempferol, quercetin, pinocembrin dimethyl ether, 5,7-dimethoxy-3-hydroxyflavanone, and 5,7-dimethoxy-3-hydroxyflavone. | [42] |
| Naringenin | Escherichia coli | Kaempferol and astragalin | [43] |
| Flavonoids (naringenin, hesperetin, luteolin, diosmetin, apigenin, genistein, formononetin, and kaempferol) | Isaria fumosorosea | Mono-methylglucosides, mono-glucoside, and di-methylglucoside | [47] |
| Tannins and gallotannins | Lactobacillus plantarum | Glucose, gallic acid, and pyrogallol | [52] |
| Tannin-rich substrates (eucalyptus leaves, pomegranate peel, banana peel, guava leaves, and wheat bran) | A. niger Trichoderma viride | Gallic acid | [55] |
| Rice bran | Aspergillus oryzae Rhizopus oryzae | Ferulic acid, 4-hydroxybenzoic acid, caffeic acid, sinapic acid, vanillic acid, and syringic acid | [56] |
| Kiwi pulp | L. plantarum | Protocatechuic chlorogenic acids (dihydroxy coumarin and p-coumaric acid) | [62] |
| Apple juice | Lactic acid bacteria (Lactobacillus spp. and Bifidobacterium spp.) | Trans-2-hexen-1-ol, 1-octanol, citronellol, geraniol, (E)-2-hexenal, and methylheptenone | [65] |
| Stevia rebaudiana | Saccharomyces cerevisiae | Sterebins O, P1, and P2 (terpenoids) | [69] |
| Illigera aromatica | Clonostachys rogersoniana | (1R*,3R*,4S*,6R*)-6,8-dihydroxymenthol, and cis-4-hydroxy-5-(1-hydroxy-1-methylethyl)-2-methyl-2-cyclohexene-1-one (terpenoids) | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omarini, A.B.; Achimón, F.; Brito, V.D.; Zygadlo, J.A. Fermentation as an Alternative Process for the Development of Bioinsecticides. Fermentation 2020, 6, 120. https://doi.org/10.3390/fermentation6040120
Omarini AB, Achimón F, Brito VD, Zygadlo JA. Fermentation as an Alternative Process for the Development of Bioinsecticides. Fermentation. 2020; 6(4):120. https://doi.org/10.3390/fermentation6040120
Chicago/Turabian StyleOmarini, Alejandra B., Fernanda Achimón, Vanessa D. Brito, and Julio A. Zygadlo. 2020. "Fermentation as an Alternative Process for the Development of Bioinsecticides" Fermentation 6, no. 4: 120. https://doi.org/10.3390/fermentation6040120
APA StyleOmarini, A. B., Achimón, F., Brito, V. D., & Zygadlo, J. A. (2020). Fermentation as an Alternative Process for the Development of Bioinsecticides. Fermentation, 6(4), 120. https://doi.org/10.3390/fermentation6040120