Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures
Abstract
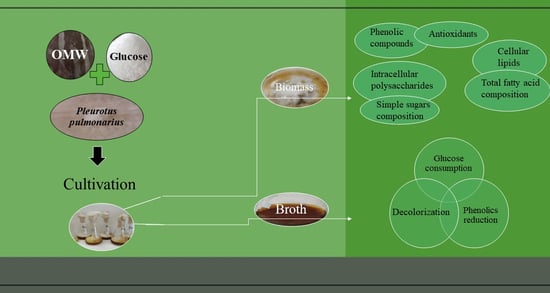
:1. Introduction
2. Materials and Methods
2.1. Fungal Species, Substrates and Culture Conditions
2.2. Analytical Methods
3. Results and Discussion
3.1. Biomass Production, Sugars Consumption
3.2. Removal of Phenolic Compounds–Decolorization
3.3. IPS Synthesis and Composition
3.4. Fungal Phenolic Compounds–Antioxidant Components Monitoring
3.5. Total Lipid Content and Fatty Acid (FA) Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mantzavinos, D.; Kalogerakis, N. Treatment of olive mill effluents Part I. Organic matter degradation by chemical and biological processes—An overview. Environ. Int. 2005, 31, 289–295. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An overview on olive mill wastes and their valorisation methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Ouzounidou, G.; Zervakis, G.; Gaitis, F. Raw and microbiologically detoxified olive mill waste and their impact on plant growth. Terr. Aquat. Environ. Toxicol. 2010, 4, 21–38. [Google Scholar]
- Crognale, S.; D’Annibale, A.; Federici, F.; Fenice, M.; Quaratino, D.; Petruccioli, M. Olive oil mill wastewater valorisation by fungi. J. Chem. Technol. Biotechnol. 2006, 81, 1547–1555. [Google Scholar] [CrossRef]
- Aggelis, G.; Iconomou, D.; Christou, M.; Bokas, D.; Kotzailias, S.; Christou, G.; Tsagou, V.; Papanikolaou, S. Phenolic removal in a model olive oil mill wastewater using Pleurotus ostreatus in bioreactor cultures and biological evaluation of the process. Water Res. 2003, 37, 3897–3904. [Google Scholar] [CrossRef]
- Blánquez, P.; Caminal, G.; Sarrà, M.; Vicent, M.T.; Gabarrell, X. Olive oil mill waste waters decoloration and detoxification in a bioreactor by the white rot fungus Phanerochaete flavido-alba. Biotechnol. Prog. 2002, 18, 660–662. [Google Scholar] [CrossRef]
- Tsioulpas, A.; Dimou, D.; Iconomou, D.; Aggelis, G. Phenolic removal in olive oil mill wastewater by strains of Pleurotus spp. in respect to their phenol oxidase (laccase) activity. Bioresour. Technol. 2002, 84, 251–257. [Google Scholar] [CrossRef]
- Zervakis, G.; Yiatras, P.; Balis, C. Edible Mushrooms from Olive Oil Mill Wastes. Int. Biodeterior. Biodegrad. 1996, 38, 237–243. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Gardeli, C.; Papanikolaou, S. Impact of olive mill wastewaters on the physiological behavior of a wild-type new Ganoderma resinaceum isolate. Environ. Sci. Pollut. Res. 2021, 28, 20570–20585. [Google Scholar] [CrossRef] [PubMed]
- Economou, C.N.; Philippoussis, A.N.; Diamantopoulou, P.A. Spent mushroom substrate for a second cultivation cycle of Pleurotus mushrooms and dephenolization of agro-industrial wastewaters. FEMS Microbiol. Lett. 2020, 367, 8. [Google Scholar] [CrossRef] [PubMed]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Merhautová, V.; Zervakis, G.I. Olive mill wastewater biodegradation potential of white-rot fungi—Mode of action of fungal culture extracts and effects of ligninolytic enzymes. Bioresour. Technol. 2015, 189, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Koutrotsios, G.; Zervakis, G.I. Comparative examination of the olive mill wastewater biodegradation process by various wood-rot macrofungi. BioMed. Res. Int. 2014, 2014, 482937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakhtar, H.; Ismaili-Alaoui, M.; Philippoussis, A.; Perraud-Gaime, I.; Roussos, S. Screening of strains of Lentinula edodes grown on model olive mill wastewater in solid and liquid state culture for polyphenol biodegradation. Int. Biodeterior. Biodegrad. 2010, 64, 167–172. [Google Scholar] [CrossRef]
- Matos, L.C.; Pereira, J.A.; Andrade, P.B.; Seabra, R.M.; Oliveira, M.B.P. Evaluation of a numerical method to predict the polyphenols content in monovarietal olive oils. Food Chem. 2007, 102, 976–983. [Google Scholar] [CrossRef]
- Morillo, J.A.; Antizar-Ladislao, B.; Monteoliva-Sánchez, M.; Ramos-Cormenzana, A.; Russell, N.J. Bioremediation and biovalorisation of olive-mill wastes. Appl. Microbiol. Biotechnol. 2008, 82, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulou, P.; Papanikolaou, S.; Kapoti, M.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part I: Screening various mushroom species. Appl. Biochem. Biotechnol. 2012, 167, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Dimou, D.M.; Georgala, A.; Komaitis, M.; Aggelis, G. Mycelial fatty acid composition of Pleurotus spp. and its application in the intrageneric differentiation. Mycol. Res. 2002, 106, 925–929. [Google Scholar] [CrossRef]
- Pedneault, K.; Angers, P.; Avis, T.J.; Gosselin, A.; Tweddell, R.J. Fatty acid profiles of polar and non-polar lipids of Pleurotus ostreatus and P. cornucopiae var. ‘citrino-pileatus’ grown at different temperatures. Mycol. Res. 2007, 111, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.-H.; Zhong, J.-J. Two-stage culture process for improved production of ganoderic acid by liquid fermentation of higher fungus Ganoderma lucidum. Biotechnol. Prog. 2002, 18, 51–54. [Google Scholar] [CrossRef]
- Fazenda, M.L.; Seviour, R.; McNeil, B.; Harvey, L.M. Submerged culture fermentation of ‘higher fungi’: The Macrofungi. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 2008; Volume 63, pp. 33–103. [Google Scholar]
- Barros, L.; Ferreira, M.-J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- Larondelle, Y.; Evers, D. Dietary Antioxidants and Oxidative Stress from a Human and Plant Perspective: A Review. Curr. Nutr. Food Sci. 2010, 6, 2–12. [Google Scholar]
- Manu-Tawiah, W.; Martin, A. Chemical composition of Pleurotus ostreatus mycelial biomass. Food Microbiol. 1987, 4, 303–310. [Google Scholar] [CrossRef]
- Dedousi, M.; Fourtaka, K.; Melanouri, E.-M.; Argyropoulos, D.; Psallida, C.; Diamantis, I.; Papanikolaou, S.; Diamantopoulou, P. Detoxification of molasses and production of mycelial mass and valuable metabolites by Morchella species. Appl. Sci. 2021, 11, 9481. [Google Scholar] [CrossRef]
- Sarris, D.; Philippoussis, A.; Mallouchos, A.; Diamantopoulou, P. Valorization of low-cost, carbon-rich substrates by edible ascomycetes and basidiomycetes grown on liquid cultures. FEMS Microbiol. Lett. 2020, 367, 20. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Aggelis, G.; Philippoussis, A. Adaptation of Volvariella volvacea metabolism in high carbon to nitrogen ratio media. Food Chem. 2016, 196, 272–280. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Katsarou, E.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Mushroom polysaccharides and lipids synthesized in liquid agitated and static cultures. Part II: Study of Volvariella volvacea. Appl. Biochem. Biotechnol. 2012, 167, 1890–1906. [Google Scholar] [CrossRef] [PubMed]
- Gern, R.M.M.; Wisbeck, E.; Rampinelli, J.R.; Ninow, J.L.; Furlan, S.A. Alternative medium for production of Pleurotus ostreatus biomass and potential antitumor polysaccharides. Bioresour. Technol. 2008, 99, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Liu, X.; Jia, L.; Song, Z.; Deng, P.; Fan, K. Optimization for the production of exopolysaccharides from Morchella esculenta SO-02 in submerged culture and its antioxidant activities in vitro. Carbohydr. Polym. 2010, 79, 700–704. [Google Scholar] [CrossRef]
- Crognale, S.; Federici, F.; Petruccioli, M. Beta-Glucan production by Botryosphaeria rhodina on undiluted olive-mill wastewaters. Biotechnol. Lett. 2004, 25, 2013–2015. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S.; Komaitis, M.; Aggelis, G.; Philippoussis, A. Patterns of major metabolites biosynthesis by different mushroom fungi grown on glucose-based submerged cultures. Bioprocess Biosyst. Eng. 2013, 37, 1385–1400. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH>• free radical method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2003, 26. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Kuswandi, B.; Hidayat, M.A. A novel high throughput method based on the DPPH• dry reagent array for determination of antioxidant activity. Food Chem. 2013, 141, 4102–4106. [Google Scholar] [CrossRef]
- Sarantou, S.; Stoforos, N.G.; Kalantzi, O.; Papanikolaou, S. Biotechnological valorization of biodiesel-derived glycerol: Trials with the non-conventional yeasts Yarrowia lipolytica and Rhodosporidium sp. Carbon Resour. Convers. 2021, 4, 61–75. [Google Scholar] [CrossRef]
- Elisashvili, V.I.; Kachlishvili, E.T.; Wasser, S.P. Carbon and nitrogen source effects on basidiomycetes exopolysaccharide production. Prikl. Biokhim. Mikrobiol. 2009, 45, 592–596. [Google Scholar] [CrossRef]
- Mulinacci, N.; Romani, A.; Galardi, C.; Pinelli, P.; Giaccherini, C.; Vincieri, F.F. Polyphenolic content in olive oil waste waters and related olive samples. J. Agric. Food Chem. 2001, 49, 3509–3514. [Google Scholar] [CrossRef] [Green Version]
- Mekki, A.; Dhouib, A.; Sayadi, S. Changes in microbial and soil properties following amendment with treated and untreated olive mill wastewater. Microbiol. Res. 2006, 161, 93–101. [Google Scholar] [CrossRef]
- Yesilada, O.; Kahraman, S.; Sam, M. Biodegradation of olive oil mill wastewater by Coriolus versicolor and Funalia trogii: Effects of agitation, initial COD concentration, inoculum size and immobilization. World J. Microbiol. Biotechnol. 1998, 14, 37–42. [Google Scholar] [CrossRef]
- Ntougias, S.; Baldrian, P.; Ehaliotis, C.; Nerud, F.; Antoniou, T.; Merhautová, V.; Zervakis, G. Biodegradation and detoxification of olive mill wastewater by selected strains of the mushroom genera Ganoderma and Pleurotus. Chemosphere 2012, 88, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Lee, J.S.; Cho, J.Y.; Kim, Y.E.; Hong, E.K. Process development for mycelial growth and polysaccharide production in Tricholoma matsutake liquid culture. J. Biosci. Bioeng. 2010, 109, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Komura, D.L.; Ruthes, A.C.; Carbonero, E.R.; Alquini, G.; Rosa, M.C.; Sassaki, G.L.; Iacomini, M. The origin of mannans found in submerged culture of basidiomycetes. Carbohydr. Polym. 2010, 79, 1052–1056. [Google Scholar] [CrossRef]
- Chang, S.C.; Steinkraus, K.H. Lignocellulolytic enzymes produced by Volvariella volvacea, the edible straw mushroom. Appl. Environ. Microbiol. 1982, 43, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.-L.; Li, W.-Q.; Wu, X.-Q.; Cheng, J.-W.; Ma, S.-Y. Statistical optimization of media for mycelial growth and exo-polysaccharide production by Lentinus edodes and a kinetic model study of two growth morphologies. Biochem. Eng. J. 2010, 49, 104–112. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Dokianakis, S.; Kornaros, M.; Aggelis, G.; Lyberatos, G. Removal of phenolics in olive mill wastewaters using the white-rot fungus Pleurotus ostreatus. Water Res. 2002, 36, 4735–4744. [Google Scholar] [CrossRef]
- Sarris, D.; Giannakis, M.; Philippoussis, A.; Komaitis, M.; Koutinas, A.A. Papanikolaou, S. Conversions of olive mill wastewater-based media by Saccharomyces cerevisiae through sterile and non-sterile bioprocesses. J. Chem. Technol. Biotechnol. 2013, 88, 958–969. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of added-value chemical compounds through bioconversions of olive-mill wastewaters blended with crude glycerol by a Yarrowia lipolytica strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.; Wang, W.; Wang, F.; Wang, Q.; Zhang, K. Polysaccharides production by submerged fermentation of Coprinus comatus and their inhibitory effects on non-enzymatic glycosylation. JMPR 2012, 6, 1375–1381. [Google Scholar]
- Fakas, S.; Galiotou-Panayotou, M.; Papanikolaou, S.; Komaitis, M.; Aggelis, G. Compositional shifts in lipid fractions during lipid turnover in Cunninghamella echinulata. Enzym. Microb. Technol. 2007, 40, 1321–1327. [Google Scholar] [CrossRef]
- Papagianni, M. Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol. Adv. 2003, 22, 189–259. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Zervakis, G.I.; Christakopoulos, P.; Topakas, E. Degradation of olive mill wastewater by the induced extracellular ligninolytic enzymes of two wood-rot fungi. J. Environ. Manag. 2017, 203, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.G.; Chang, S.-T. Mushrooms: Cultivation, Nutritional Value, Medicinal Effect, and Environmental Impact; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Bano, Z.; Rajarathnam, S. Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr. 1988, 27, 87–158. [Google Scholar] [CrossRef] [PubMed]
- Philippoussis, A.; Diamantopoulou, P.; Israilides, C. Productivity of agricultural residues used for the cultivation of the medicinal fungus Lentinula edodes. Int. Biodeterior. Biodegrad. 2007, 59, 216–219. [Google Scholar] [CrossRef]
- Seviour, R.J.; Stasinopoulos, S.J.; Auer, D.P.; Gibbs, P.A. Production of Pullulan and other Exopolysaccharides by Filamentous Fungi. Crit. Rev. Biotechnol. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhang, W.; Liu, R.-S.; Zhu, L.-W.; Zhong, J.-J. Scale-up study on the fed-batch fermentation of Ganoderma lucidum for the hyperproduction of ganoderic acid and Ganoderma polysaccharides. Process Biochem. 2011, 46, 404–408. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, L.-L.; Li, D.-S.; Mi, Z.-Y.; Li, H.-M. Significance of inoculation density and carbon source on the mycelial growth and Tuber polysaccharides production by submerged fermentation of Chinese truffle Tuber sinense. Process Biochem. 2008, 43, 576–586. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, D.; Wan, W.; Hu, X.; Qi, Y.; Liang, Z. Enhanced simultaneous production of mycelia and intracellular polysaccharide in submerged cultivation of Cordyceps jiangxiensis using desirability functions. Process. Biochem. 2006, 41, 1887–1893. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Galiotou-Panayotou, M.; Kalantzi, O.; Aggelis, G. Modelling of simultaneous production of polygalacturonase and exopolysaccharide by Aureobasidium pullulans ATHUM 2915. Antonie Van Leeuwenhoek 1998, 73, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Ratledge, C.; Wynn, J.P. The Biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [Google Scholar] [PubMed]
- Zhong, J.-J. Tang, Y.-J. Submerged cultivation of medicinal mushrooms for production of valuable bioactive metabolites. In Biomanufacturing; Zhong, J.-J., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 25–59. [Google Scholar]
- Smiderle, F.; Olsen, L.; Ruthes, A.; Czelusniak, P.; de Santana-Filho, A.P.; Sassaki, G.; Gorin, P.; Iacomini, M. Exopolysaccharides, proteins and lipids in Pleurotus pulmonarius submerged culture using different carbon sources. Carbohydr. Polym. 2011, 87, 368–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, W.Y.; Park, Y.; Ahn, J.K.; Ka, K.H.; Park, S.Y. Factors influencing the production of endopolysaccharide and exopolysaccharide from Ganoderma applanatum. Enzym. Microb. Technol. 2007, 40, 249–254. [Google Scholar] [CrossRef]
- Sulistiany, H.; Sudirman, L.I.; Dharmaputra, O.S. Production of fruiting body and antioxidant activity of wild Pleurotus. HAYATI J. Biosci. 2016, 23, 191–195. [Google Scholar] [CrossRef]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs SM, N.; Somasundaram, R. Antioxidant activity of indigenous edible mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True morels (Morchella)—nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Jeena, G.S.; Punatha, H.; Prakash, O.; Chandra, M.; Kushwaha, K.P.S. Study on in vitro antioxidant potential of some cultivated Pleurotus species (Oyster mushroom). Indian J. Nat. Prod. Resour. IJNPR Former. Nat. Prod. Radiance NPR 2016, 5, 56–61. [Google Scholar]
- Miric, O.; Lalic, V.Z.; Miletic, D.I. The composition of some lipid fractions (phospholipids, triglycerides, free fatty acids, sterols) of wild growing edible mushrooms. Hrana I Ishr. 1985, 26, 123–128. [Google Scholar]
- Kavishree, S.; Hemavathy, J.; Lokesh, B.R.; Shashirekha, M.N.; Rajarathnam, S. Fat and fatty acids of Indian edible mushrooms. Food Chem. 2008, 106, 597–602. [Google Scholar] [CrossRef]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvador, M.; Dillon, A.J.P. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Sancholle, M.; Lösel, D.M.; Laruelle, E. Lipids in fungal biotechnology. In The Mycota II. Genetic Biotechnology; Springer: Berlin/Heidelberg, Germany, 2004; pp. 391–423. [Google Scholar]
- Hiroi, M.; Tsuyuki, H. Comparison of fatty acid composition in fruit body and spore of mushrooms (edible fungi). Bull. Coll. Agric. Vet. Med.—Nihon Univ. Jpn. 1988, 45, 104–109. [Google Scholar]
- Ratledge, C. Lipids and their metabolism. Yeasts 1989, 3, 428. [Google Scholar]
- Sumner, J.L. The fatty acid composition of basidiomycetes. New Zealand J. Bot. 1973, 11, 435–442. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Weng, C.-C.; Huang, S.-J.; Chen, C.-C.; Mau, J.-L. Nonvolatile taste components of Grifola frondosa, Morchella esculenta and Termitomyces albuminosus mycelia. LWT Food Sci. Technol. 2006, 39, 1066–1071. [Google Scholar] [CrossRef]
- Tseng, Y.-H.; Lee, Y.-L.; Li, R.-C.; Mau, J.-L. Non-volatile flavour components of Ganoderma tsugae. Food Chem. 2005, 90, 409–415. [Google Scholar] [CrossRef]
- Byrne, P.F.S.; Brennan, P.J. The Lipids of Agaricus bisporus. J. Gen. Microbiol. 1975, 89, 245–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, N.G.; Holley, M.P.; Song, C.H.; Cho, K.Y. Lipid metabolism of Pleurotus sajor caju. Ann. Appl. Biol. 1990, 116, 455–462. [Google Scholar] [CrossRef]
- Nair, N.G.; Song, C.H.; Jiang, J.Y.; Vine, J.H.; Tattum, B.; Cho, K.Y. Lipid profile of Pleurotus sajor caju. Ann. Appl. Biol. 1989, 114, 167–176. [Google Scholar] [CrossRef]
- Wild, R.; Patil, S.; Popović, M.; Zappi, M.; Dufreche, S.; Bajpai, R. Lipids from Lipomyces starkeyi. Food Technol. Biotechnol. 2010, 48, 329–335. [Google Scholar]
- Bellou, S.; Makri, A.; Sarris, D.; Michos, K.; Rentoumi, P.; Celik, A.; Papanikolaou, S.; Aggelis, G. The olive mill wastewater as substrate for single cell oil production by Zygomycetes. J. Biotechnol. 2014, 170, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Sarris, D.; Galiotou-Panayotou, M.; Koutinas, A.A.; Komaitis, M.; Papanikolaou, S. Citric acid, biomass and cellular lipid production by Yarrowia lipolytica strains cultivated on olive mill wastewater-based media. J. Chem. Technol. Biotechnol. 2011, 86, 1439–1448. [Google Scholar] [CrossRef]
- Bespalova, L.A.; Makarov, O.E.; Antonyuk, L.P.; Ignatov, V.V. Lipogenesis in the Basidiomycetes Pleurotus ostreatus and Flammulina velutipes cultivated on different Media. Appl. Biochem. Microbiol. 2002, 38, 349–354. [Google Scholar] [CrossRef]
- Ribeiro, B.; de Pinho, P.G.; Andrade, P.; Baptista, P.; Valentão, P. Fatty acid composition of wild edible mushrooms species: A comparative study. Microchem. J. 2009, 93, 29–35. [Google Scholar] [CrossRef] [Green Version]
- Solomko, E.F.; Panchenko, L.P.; Sil’chenkova, R.K. Lipid content and fatty acid composition of the higher edible fungus—The oyster mushroom Pleurotus ostreatus (Fr.) Kummer. Prikl. Biokhim. Mikrobiol. 1984, 20, 273–279. [Google Scholar] [PubMed]
- Erwin, J. Lipids and Biomembranes of Eukaryotic Microorganisms; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]










| Characteristics of OMW | |
|---|---|
| Total sugars | 30.0 g/L |
| Phenolic compounds | 11.5 g/L |
| Free amino nitrogen | 70.5 mg/L |
| Proteins | 0.5 g/L |
| pH | 4.95 |
| EC | 13.86 mS/cm |
| OMW Concentration (g/L) | Glucose Concentration (g/L) | Culture Media |
|---|---|---|
| 0 | 40 | S0-G40 |
| 60 | S0-G60 | |
| 1 | 40 | S1-G40 |
| 60 | S1-G60 | |
| 2 | 40 | S2-G40 |
| 60 | S2-G60 | |
| 3 | 40 | S3-G40 |
| 60 | S3-G60 |
| Culture Media * | Time (Day) | Χ (g/L) | Glccons (g/L) | YX/S (%, w/w) | IPS (g/L) | YIPS/X (%, w/w) | L (g/L) | YL/X (%, w/w) | |
|---|---|---|---|---|---|---|---|---|---|
| S0-G40 | a, d | 43 | 14.53 ± 0.04 | 18.83 ± 0.25 | 0.77 ± 0.02 | 2.08 ± 0.12 | 14.34 ± 0.75 | 0.94 ± 0.08 | 6.47 ± 0.10 |
| b, c | 37 | 14.44 ± 0.05 | 17.86 ± 0.29 | 0.80 ± 0.01 | 3.25 ± 0.20 | 22.51 ± 0.85 | 0.51 ± 0.06 | 3.53 ± 0.12 | |
| S0-G60 | a, b, d | 43 | 19.62 ± 0.56 | 30.09 ± 0.32 | 0.65 ± 0.02 | 4.01 ± 0.05 | 20.41 ± 0.12 | 1.63 ± 0.05 | 8.31 ± 0.09 |
| c | 37 | 18.29 ± 0.26 | 29.14 ± 0.35 | 0.63 ± 0.01 | 3.84 ± 0.04 | 20.99 ± 0.16 | 0.80 ± 0.06 | 4.37 ± 0.09 | |
| S1-G40 | a, d | 43 | 22.66 ± 0.12 | 29.22 ± 0.31 | 0.78± 0.01 | 2.83 ± 0.22 | 12.79 ± 0.52 | 1.38 ± 0.04 | 6.09 ± 0.06 |
| b, c | 37 | 19.51 ± 0.56 | 24.84 ± 0.35 | 0.79 ± 0.02 | 3.92 ± 0.21 | 20.08 ± 0.63 | 0.93 ± 0.04 | 4.77 ± 0.11 | |
| S1-G60 | a, d | 43 | 24.39 ± 0.58 | 31.35 ± 0.58 | 0.78 ± 0.01 | 3.44 ± 0.12 | 14.09 ± 0.28 | 2.85 ± 0.07 | 11.69 ± 0.14 |
| b, c | 29 | 21.73 ± 0.06 | 27.41 ± 0.62 | 0.79 ± 0.01 | 4.03 ± 0.18 | 18.55 ± 0.95 | 1.16 ± 0.11 | 5.34 ± 0.03 | |
| S2-G40 | a, d | 43 | 25.11 ± 0.71 | 32.53 ± 0.21 | 0.77 ± 0.01 | 3.10 ± 0.22 | 12.35 ± 0.64 | 2.30 ± 0.11 | 9.16 ± 0.12 |
| b, c | 37 | 24.86 ± 0.62 | 31.59 ± 0.23 | 0.79 ± 0.01 | 4.32 ± 0.14 | 17.39 ± 1.01 | 1.94 ± 0.09 | 7.80 ± 0.23 | |
| S2-G60 | a, b, d | 43 | 29.82 ± 0.76 | 41.65 ± 0.14 | 0.72 ± 0.01 | 4.38 ± 0.09 | 14.70 ± 0.05 | 2.08 ± 0.13 | 6.98 ± 0.21 |
| c | 37 | 25.14 ± 0.52 | 33.71 ± 0.12 | 0.74 ± 0.01 | 4.06 ± 0.07 | 16.15 ± 0.87 | 1.24 ± 0.12 | 4.93 ± 0.35 | |
| S3-G40 | a, b, d | 43 | 30.61 ± 0.73 | 35.43 ± 0.32 | 0.88 ± 0.03 | 4.30 ± 0.12 | 14.04 ± 0.14 | 2.19 ± 0.12 | 7.15 ± 0.05 |
| c | 37 | 26.57 ± 0.73 | 34.69 ± 0.11 | 0.76 ± 0.02 | 3.98 ± 0.09 | 14.98 ± 0.12 | 1.87 ± 0.12 | 6.96 ± 0.02 | |
| S3-G60 | a, b, d | 43 | 32.76 ± 0.53 | 42.07 ± 0.24 | 0.78 ± 0.01 | 3.63 ± 0.05 | 11.01 ± 0.09 | 2.00 ± 0.09 | 6.11 ± 0.21 |
| c | 37 | 28.86 ± 0.45 | 37.63 ± 0.21 | 0.77 ± 0.01 | 3.50 ± 0.02 | 12.13 ± 0.04 | 1.70 ± 0.08 | 5.89 ± 0.32 |
| Culture Media | Day | Glucose (%) | Mannitol (%) |
|---|---|---|---|
| S0-G40 | 17 | 83.24 | 16.76 |
| 29 | 88.14 | 11.86 | |
| 43 | 89.20 | 10.80 | |
| S0-G60 | 17 | 84.15 | 15.85 |
| 29 | 87.32 | 12.68 | |
| 43 | 88.96 | 11.04 | |
| S1-G40 | 17 | 85.28 | 14.72 |
| 29 | 82.21 | 17.79 | |
| 43 | 78.31 | 21.69 | |
| S1-G60 | 17 | 89.36 | 10.64 |
| 29 | 84.85 | 15.15 | |
| 43 | 78.45 | 21.55 | |
| S2-G40 | 17 | 89.24 | 10.76 |
| 29 | 85.56 | 14.44 | |
| 43 | 79.23 | 20.77 | |
| S2-G60 | 17 | 88.49 | 11.51 |
| 29 | 86.35 | 13.65 | |
| 43 | 80.75 | 19.25 | |
| S3-G40 | 17 | 88.28 | 11.72 |
| 29 | 85.21 | 14.79 | |
| 43 | 76.52 | 23.48 | |
| S3-G60 | 17 | 87.21 | 12.79 |
| 29 | 82.65 | 17.35 | |
| 43 | 75.32 | 24.68 |
| Fatty Acids (%, w/w) | Culture Media * | |||||||
|---|---|---|---|---|---|---|---|---|
| S0-G40 | SO-G60 | S1-G40 | S1-G60 | S2-G40 | S2-G60 | S3-G40 | S3-G60 | |
| Undecanoic acid (C11:0) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Lauric acid (C12:0) | 2.27 | 0.00 | 0.00 | 2.72 | 0.18 | 0.00 | 0.20 | 0.18 |
| Myristic acid (C14:0) | 3.19 | 1.93 | 0.43 | 0.38 | 0.31 | 0.62 | 0.32 | 0.35 |
| Pentadecenoic acid (C15:1) | 7.32 | 9.17 | 0.00 | 0.00 | 0.39 | 0.00 | 0.35 | 0.00 |
| Palmitic acid (C16:0) | 23.10 | 22.30 | 16.50 | 7.78 | 14.70 | 18.23 | 14.00 | 14.80 |
| Palmitoleic acid (C16:1) | 1.42 | 1.15 | 0.00 | 0.00 | 0.45 | 0.00 | 0.45 | 0.48 |
| Heptadecanoic acid (C17:0) | 0.92 | 0.00 | 0.00 | 0.26 | 0.25 | 0.00 | 0.23 | 0.25 |
| Stearic acid (C18:0) | 10.20 | 9.85 | 4.30 | 4.25 | 5.05 | 4.90 | 4.98 | 4.83 |
| Oleic acid (C18:1) | 11.80 | 16.90 | 27.30 | 29.80 | 28.60 | 27.50 | 28.84 | 29.60 |
| Octadecenoic Acid (C18:1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.22 | 0.37 |
| Linoleic acid (C18:2) | 27.42 | 28.55 | 47.43 | 46.10 | 41.75 | 46.86 | 41.08 | 41.95 |
| α-Linolenic acid (C18:3) | 0.00 | 0.00 | 0.00 | 0.23 | 0.20 | 0.26 | 0.26 | 0.28 |
| Arachidic acid (C20:0) | 0.00 | 0.00 | 0.40 | 0.30 | 0.50 | 0.00 | 0.71 | 0.69 |
| Eicosenoic acid (C20:1) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Behenic acid (C22:0) | 0.00 | 0.00 | 0.41 | 0.43 | 0.69 | 0.00 | 0.90 | 0.51 |
| Saturated | 39.68 | 34.08 | 22.08 | 16.12 | 21.68 | 23.75 | 21.34 | 21.61 |
| Monounsaturated | 20.54 | 27.22 | 27.30 | 29.80 | 29.69 | 27.50 | 29.86 | 30.45 |
| Polyunsaturated | 27.42 | 28.55 | 47.43 | 46.33 | 41.95 | 47.12 | 41.34 | 42.23 |
| U.I. | 0.91 | 0.97 | 1.39 | 1.30 | 1.28 | 1.40 | 1.26 | 1.30 |
| Other acids | 7.26 | 3.65 | 0.88 | 1.80 | 1.89 | 0.81 | 2.94 | 1.85 |
| Not identified peaks (35 min–38.5 min) | 5.09 | 6.50 | 2.34 | 5.95 | 4.80 | 0.81 | 4.52 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diamantis, I.; Melanouri, E.-M.; Dedousi, M.; Panagopoulou, I.; Papanikolaou, S.; Stoforos, N.G.; Diamantopoulou, P. Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures. Fermentation 2022, 8, 129. https://doi.org/10.3390/fermentation8030129
Diamantis I, Melanouri E-M, Dedousi M, Panagopoulou I, Papanikolaou S, Stoforos NG, Diamantopoulou P. Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures. Fermentation. 2022; 8(3):129. https://doi.org/10.3390/fermentation8030129
Chicago/Turabian StyleDiamantis, Ilias, Eirini-Maria Melanouri, Marianna Dedousi, Ioanna Panagopoulou, Seraphim Papanikolaou, Nikolaos G. Stoforos, and Panagiota Diamantopoulou. 2022. "Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures" Fermentation 8, no. 3: 129. https://doi.org/10.3390/fermentation8030129
APA StyleDiamantis, I., Melanouri, E. -M., Dedousi, M., Panagopoulou, I., Papanikolaou, S., Stoforos, N. G., & Diamantopoulou, P. (2022). Sustainable and Eco-Friendly Conversions of Olive Mill Wastewater-Based Media by Pleurotus pulmonarius Cultures. Fermentation, 8(3), 129. https://doi.org/10.3390/fermentation8030129