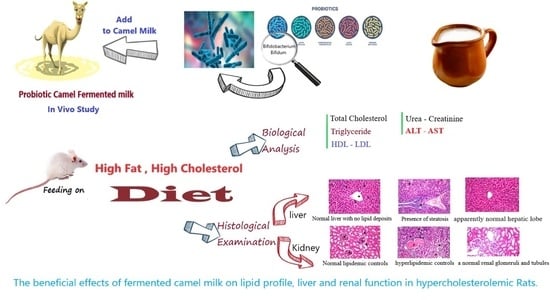
Beneficial Effects of Fermented Camel and Cow’s Milk in Lipid Profile, Liver, and Renal Function in Hypercholesterolemic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Experimental Animals
2.3. High-Fat, High-Cholesterol Diet
2.4. Chemicals
2.5. Starter Cultures and Fermented Milk Manufacture
2.6. Organoleptic Properties
2.7. Chemical Analysis
2.8. Experimental Design
2.9. Biochemical Analysis
2.10. Histopathological Examination
2.11. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Various Fermented Milk
3.2. Sensory Evaluation of Different Fermented Milks
3.3. Effect of Fermented Milk on the Body Weight Gain in Rats
3.4. Effect of Fermented Milks on Blood Lipid Profile
3.5. Effect of Different Fermented Milks on Liver Functions
3.6. Effect of Fermented Milks on Renal Function in Hypercholesterolemic Rats
3.7. Histological Changes in Experimental Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izadi, A.; Khedmat, L.; Mojtahedi, S.Y. Nutritional and therapeutic perspectives of camel’s milk and its protein hydrolysates: A review on versatile bio functional properties. J. Funct. Foods 2019, 60, 103441. [Google Scholar] [CrossRef]
- Ayoub, M.A.; Palakkott, A.R.; Ashraf, A.; Iratni, R. The molecular basis of the antidiabetic properties of camel’s milk. Diabetes Res. Clin. Pract. 2018, 146, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Ejtahed, H.S.; Angoorani, P.; Eslami, F.; Azizi, F. Camel milk has beneficial effects on diabetes mellitus: A systematic review. Int. J. Endocrinol. Metab. 2017, 15, 42150–42158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solanki, D.; Hati, S. Fermented camel milk: A Review on its bio-functional properties. Emir. J. Food Agric. 2018, 30, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Tak, L.; Bais, B.; Singh, R.; Singh, S.; Nayak, T. Assessment of probiotic and neutraceutical properties of camel milk yoghurt. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3351–3357. [Google Scholar] [CrossRef]
- Ali, A.A.; Alyan, A.A.; Bahobail, A.S. Effect of fermented camel milk and cow milk containing (Bifidobacteria) enriched diet in rats fed on cholesterol level. Agric. Sci. Res. J. 2013, 3, 342–346. [Google Scholar]
- Devendra, K.; Verma, K.A.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel’s milk: Alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016, 46, 217–227. [Google Scholar] [CrossRef]
- Kimoto-Nira, H.; Mizumachi, K.; Kobayashi, M.N.M.; Fujita, Y.; Okamoto, T.; Suzuki, I.; Tsuji, N.M.; Kurisaki, J.I.; Ohmomo, S. Lactococcus sp. as potential probiotic lactic acid bacteria. J. Agric. Res. Quart. 2007, 41, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Yahya, M.H.; Alhaj, O.A.; AL-Khalifah, A.S.; Ahmad, T.; Almnaizel, A.T. Hypocholesterolemic effect of camel milk on rats fed a high-cholesterol diet. Emir. J. Food Agric. 2018, 30, 288–294. [Google Scholar] [CrossRef]
- Tripathi, M.; Giri, S. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Walsh, H.; Ross, J.; Hendricks, G.; Mingruo, G. Physico-chemical properties, probiotic survivability, microstructure, and acceptability of a yogurt-like symbiotic oats-based product using pre-polymerized whey protein as a gelation agent. J. Food Sci. 2010, 75, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Grover, S.; Batish, V.K. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br. J. Nutr. 2011, 105, 561–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, D.R.; Chawan, C.B.; Pulusani, S.R. Influence of milk and thermophilus milk on plasma cholesterol levels and hepatic cholesterogenesis in rates. J. Food Sci. 1981, 46, 1339–1341. [Google Scholar] [CrossRef]
- Scholz-Ahrens, K.E.; Ade, P.; Marten, B.; Weber, P.; Timm, W.; Asil, Y.; Gluer, C.; Schrezenmeir, J. Prebiotics, probiotics, and symbiotic affect mineral absorption, bone mineral content, and bone structure. J. Nutr. 2007, 137, 838–846. [Google Scholar] [CrossRef]
- Zaid, A. Study the effect of probiotic bacteria isolated from foods on pathogens. Biomed. Res. 2018, 12, 2509–2515. [Google Scholar] [CrossRef]
- Zhang, M.; Hang, X.; Fan, X.; Li, D.; Yang, H. Characterization and selection of Lactobacillus strains for their effect on bile tolerance, taurocholate deconjugation and cholesterol removal. World J. Microbiol. Biotechnol. 2008, 24, 7–14. [Google Scholar] [CrossRef]
- Kiortsis, D.N.; Filippatos, T.D.; Mikhailidis, D.P.; Elisaf, M.S.; Liberopoulos, E.N. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis 2007, 195, 7–16. [Google Scholar] [CrossRef]
- Kumar, M.; Rakesh, S.; Nagpal, R.; Hemalatha, R.; Ramakrishna, A.; Sudarshan, V.; Ramagoni, R.; Shujauddin, M.; Verma, V.; Kumar, A.; et al. Probiotic Lactobacillus rhamnosus GG and Aloe vera gel improve lipid profiles in hypercholesterolemic rats. Nutrition 2013, 29, 574–579. [Google Scholar] [CrossRef]
- El-Zahar, K.H.; Hassan, F.Y.; Al-Qaba, S.F. Protective effect of fermented camel and cow milk containing Bifidobacterium longum BB 536 on blood lipids profile in hypercholesterolemic rats. J. Nutr. Metab. 2021, 2021, 1–12. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Tamime, A.Y.; Robinson, R.K. Yoghurt: Science and Technology, 2nd ed.; Woodhead Publishing Ltd.: Cambridge, UK, 1999; pp. 420–432. ISBN 0-8493-1785-1. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis, 20th ed.; Latimer, G.W., Jr., Ed.; AOAC International: Rockville, MD, USA, 2016; ISBN 0935584870. [Google Scholar]
- Armbruster, D.A.; Lambert, P.A. Direct assay of LDL Cholesterol: Comparing measurement and calculation. Lab. Med. 1996, 27, 613–617. [Google Scholar] [CrossRef] [Green Version]
- Schumann, G.I.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Young, D.S.; Friedman, R.B. Effects of Disease on Clinical Laboratory Tests, 4th ed.; AACC Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Banchroft, J.D.; Alton, D.; Floyd, G. Theory and Practice of Histological Techniques, 7th ed.; Elsevier Health Science: New York, NY, USA, 2013. [Google Scholar]
- Ibrahem, S.A.; El Zubeir, I.E.M. Processing, composition and sensory characteristic of yoghurt made from camel milk and camel-sheep milk mixtures. Small Rumin. Res. 2016, 136, 109–112. [Google Scholar] [CrossRef]
- Galeboe, O.; Seifu, E.; Sekwati-Monang, B. Production of Camel Milk Yoghurt: Physicochemical and Microbiological Quality and Consumer Acceptability. Int. J. Food Stud. 2018, 7, 51–63. [Google Scholar] [CrossRef]
- Eissa, E.A.; Yagoub, A.A.; Babiker, E.E.; Ahmed, I.A.M. Physicochemical, microbiological and sensory characteristics of yoghurt produced from camel milk during storage. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2305–2313. [Google Scholar]
- Boukria, O.; El-Hadrami, E.; Sameen, A.; Sahar, A.; Khan, S.; Safarov, J.; Sultanova, S.; Leriche, F.; Aït-Kaddour, A. Biochemical, physicochemical and sensory properties of yoghurts made from mixing milks of different mammalian species. Foods 2020, 9, 1722. [Google Scholar] [CrossRef]
- Shirani, F.; Teimoori, A.; Rashno, M.; Latifi, S.M.; Karandish, M. Using rats as a research model to investigate the effect of human adenovirus 36 on weight gain. ARYA Atheroscler. 2017, 13, 167–171. [Google Scholar]
- Zhang, J.; Xiao, X.; Xu, T.; Wu, F. Dietary supplementation with Lactobacillus plantarum dy-1 fermented barley suppresses body weight gain in high-fat diet-induced obese rats. J. Sci. Food Agric. 2016, 96, 4907–4917. [Google Scholar] [CrossRef]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Hypocholesterolaemic effect of yoghurt containing Bif. pseudocatenulatum G4 or Bif. longum BB536. Food Chem. 2018, 135, 356–361. [Google Scholar] [CrossRef]
- Abdelgawad, I.A.; El-Sayeda, E.M.; Hafez, B.S.A.; El-Zeinia, H.M.; Saleh, F.A. The hypercholesterolaemic effect of milk yoghurt and soy-yoghurt containing Bifidobacteria in rats fed on a cholesterol-enriched diet. Int. Dairy J. 2005, 15, 37–44. [Google Scholar] [CrossRef]
- Adriani, L.; Andiany, A.; Latipudin, D.; Benito, T.; Cahyani, C. Effect of fermented cow and soybean milk with probiotic in improving blood cholesterol and triglyceride levels on broilers. Int. J. Poult. Sci. 2018, 17, 600–604. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for cholesterol-lowering probiotics from lactic acid bacteria isolated from corn silage based on three hypothesized pathways. Int. J. Mol. Sci. 2019, 20, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chobert, J.M.; El-Zahar, K.H.; Sitohy, M.Z.; Dalgalarrondo, M.; Métro, F.; Choiset, Y.; Haertlé, T. Angiotensin I-converting-enzyme (ACE)- inhibitory activity of tryptic peptides of ovine β-lactoglobulin and of milk yoghurts obtained by using different starters. Lait 2005, 85, 141–152. [Google Scholar] [CrossRef]
- El-Zahar, K.; Abd-El-Zaher, A.M.; Bassiony, H.E. Effect of probiotic yoghurt on some metabolic parameters in hypercholesterolemic rats. Wulfenia J. 2014, 21, 57–79. [Google Scholar]
- Nabavi, S.; Rafraf, M.; Somi, H.M.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Abdel-Haleem, S.A.; Ibrahima, A.Y.; Ismaila, R.F.; Shaffieb, N.M.; Hendawya, S.F.; Omer, E.A. In-vivo hypoglycemic and hypolipidemic properties of Tagetes lucidaalcoholic extract in streptozotocin-induced hyperglycemic Wistar albino rat. Ann. Agric. Sci. 2017, 62, 169–181. [Google Scholar] [CrossRef]
- Osman, N.; Adawi, D.; Ahrné, S.; Jeppsson, B.; Molin, G. Endotoxin and -galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig. Liver Dis. 2007, 39, 849–856. [Google Scholar] [CrossRef]
- Darwish, H.A.; Abd-Raboh, R.N.; Mahdy, A. Camel’s milk alleviates alcohol-induced liver injury in rats. Food Chem. Toxicol. 2012, 50, 1377–1383. [Google Scholar] [CrossRef]
- Matos, S.L.; Paula, H.D.; Pedrosa, M.L.; Santos, R.C.D.; Oliveira, E.L.D.; Junior, D.A.C.; Silva, M.E. Dietary models for inducing hypercholesterolemia in rats. Braz. Arch. Biol. Tech. 2005, 48, 203–209. [Google Scholar] [CrossRef] [Green Version]
- Al-Shehri, E.A. The impact of four potential herbal foods on modifying metabolic Parameters in hypercholesterolemic rat’s model. Aust. J. Basic Appl. Sci. 2012, 6, 700–708. [Google Scholar]
- Khiralla, G.M.; Salem, S.A. Biological evaluation of wheat-salty extract, milk-wheat solution and fermented soymilk for treatment of castor oil induced diarrhea in rats. J. Food Res. 2012, 1, 298–307. [Google Scholar] [CrossRef] [Green Version]
- Trovato, A.; Taviano, M.F.; Pergolizzi, S.; Campolo, L.; De-Pasquale, R.; Miceli, N. Citrus bergamia Risso and Poiteau juice protects against renal injury of diet-induced hypercholesterolemia in rats. Phytother. Res. 2010, 24, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Omnia, M.A.; Nabila, M.A.; Nadia, R.R. Biochemical effects of propolis and bee pollen in experimentally induced hyperammonemia in rats. Benha Vet. Med. J. 2014, 27, 8–24. [Google Scholar]
- Zargar, S.; Alonazi, M.; Rizwana, H.; Wani, T.A. Resveratrol reverses thioacetamide-induced renal assault with respect to oxidative stress, renal function, DNA damage, and cytokine release in Wistar rats. Oxid. Med. Cell. Longev. 2019, 2019, 1702959. [Google Scholar] [CrossRef] [Green Version]
- Wa, Y.; Yin, B.; He, Y.; Xi, W.; Huang, Y.; Wang, C.; Guo, F.; Gu, R. Effects of single probiotic and combined probiotic-fermented milk on lipid metabolism in hyperlipidemic rats. Front. Microbiol. 2019, 10, 1312–1320. [Google Scholar] [CrossRef] [Green Version]
- Aleisa, A.M.; Abouhashish, H.M.; Ahmed, M.M.; Al-Rejaie, S.S.; Alkhamees, O.A.; Alroujayee, A.S. Ameliorative effects of rutin and ascorbic acid combination on hypercholesterolemia induced hepatotoxicity in female rats. Afr. J. Pharm. Pharmacol. 2013, 7, 280–288. [Google Scholar] [CrossRef] [Green Version]





| Attribute | Fermented Milk Types | |||||
|---|---|---|---|---|---|---|
| Co-T | Co-P | Ca-T | Ca-P | Ca_Co-T | Ca_Co-P | |
| Moisture (%) | 85.66 ± 1.2 b | 85.00 ± 1.2 b | 87.48 ± 1.3 a | 87.37 ± 1.3 a | 86.62 ± 1.3 a,b | 86.46 ± 1.4 a,b |
| Fat (%) | 4.80 ± 0.33 a | 4.60 ± 0.33a | 3.3 ± 00.16 c | 3.50 ± 0.20 c | 4.35 ± 0.12 b | 4.50 ± 0.33 b |
| Protein (%) | 4.83 ± 0.21 a | 4.63 ± 0.21 a | 4.39 ± 0.08 b | 4.40 ± 0.10 b | 4.47 ± 0.14 a,b | 4.49 ± 0.24 a,b |
| Ash (%) | 0.78 ± 0.07 b,c | 0.75 ± 0.07 c | 0.86 ± 0.10 a | 0.84 ± 0.10 a | 0.80 ± 0.08 b | 0.82 ± 0.09 a,b |
| Lactose (%) | 3.93 ± 0.17 a,b | 3.83 ± 0.17 b | 3.97 ± 0.22 a | 3.89 ± 0.32 b | 3.76 ± 0.16 c | 3.73 ± 0.14 c |
| pH | 4.83 ± 0.10 a | 4.73 ± 0.10 b,c | 4.71 ± 0.13 b,c | 4.76 ± 0.12 b | 4.72 ± 0.12 b,c | 4.69 ± 0.09 c |
| Acidity (%) | 0.78 ± 0.11 a | 0.75 ± 0.11 a,b | 0.71 ± 0.09 b | 0.74 ± 0.11 a,b | 0.75 ± 0.11 a,b | 0.70 ± 0.11 b |
| Attribute | Fermented Milk Types | |||||
|---|---|---|---|---|---|---|
| Co-T | Co-P | Ca-T | Ca-P | Ca_Co-T | Ca_Co-P | |
| Flavor (30) | 28.91 ± 0.3 a | 28.61 ± 0.3 a | 27 ± 0.56 b,c | 26.8 ± 0.24 c | 28.13 ± 0.78 b | 28.5 ± 0.63 a |
| Color (10) | 9.2 ± 0.12 a | 9.2 ± 0.12 a | 9.1 ± 0.17 a,b | 8.85 ± 0.19 c | 9.0 ± 0.2 b | 9.1 ± 0.24 a,b |
| Body and Texture (40) | 36.9 ± 0.4 a | 36.1 ± 0.4 b | 34.17 ± 0.82 c | 33.52 ± 0.27 d | 36.5 ± 0.24 a,b | 36.0 ± 0.33 b |
| Acidity (10) | 9.02 ± 0.2 a | 8.92 ± 0.2 a,b | 8.62 ± 0.16 b,c | 8.54 ± 0.1 c | 8.58 ± 0.18 c | 8.81 ± 0.16 b |
| Overall acceptability (10) | 9.11 ± 0.13 a | 9.01 ± 0.13 a,b | 8.64 ± 0.17 c | 8.65 ± 0.12 c | 8.9 ± 0.21 b | 8.92 ± 0.23 b |
| Total (100) | 93.14 ± 0.2 a | 91.84 ± 0.2 b | 87.53 ± 0.38 c | 86.36 ± 0.24 c | 91.11 ± 0.4 b,c | 91.33 ± 0.33 b |
| Groups | Initial Body Weight (g) | Final Body Weight (g) | Body Weight Gain (%) |
|---|---|---|---|
| NC | 265.4 ± 11.7 c | 319.3 ± 12.7 c | 20.38 ± 0.2 b,c |
| PC | 287.3 ± 11.9 a,b | 381.3 ± 13.7 a | 32.71 ± 1.62 a |
| Ca-T | 291.33 ± 10 a,b | 352.3 ± 11.9 b | 20.97 ± 0.5 b,c |
| Ca-P | 290.2 ± 12.8 a | 349.3 ± 12.6 b | 20.34 ± 0.3 b,c |
| Co-T | 285.2 ± 10.3 a,b | 347.3 ± 7.4 b | 21.71 ± 0.8 b,c |
| Co-P | 295.3 ± 9.3 a | 346.5 ± 6.4 b,c | 17.29 ± 0.8 c |
| Ca_Co-T | 279.2 ± 11.2 b | 345.7 ± 10.8 b,c | 23.66 ± 0.7 b |
| Ca_Co-P | 290.7 ± 10.2 a | 361.9 ± 10.5 a,b | 24.75 ± 0.9 b |
| Groups | TG mg/dL | TC mg/dL | HDL mg/dL | LDL mg/dL | VLDL mg/dL |
|---|---|---|---|---|---|
| NC | 43.6 ± 1.1 e | 77.6 ± 1.7 f | 41.7 ± 0.8 a | 31.2 ± 1.3 e | 12.2 ± 0.1 e |
| PC | 159.2 ± 2.1 a | 195.4 ± 1.2 a | 30.5 ± 0.4 d | 133.9 ± 1.7 a | 34.3 ± 0.5 a |
| Ca-T | 86.5 ± 1.5 c | 102.9 ± 2.2 c | 33.4 ± 1.5 c | 47.9 ± 1.6 c | 19.5 ± 0.3 c |
| Ca-P | 84.9 ± 1.1 c | 97.1 ± 1.6 c,d | 36.2 ± 1.7 b | 44.8 ± 1.6 c | 19.2 ± 0.4 c |
| Co-T | 101.9 ± 1.3 b | 113.3 ± 2.6 b | 33.7 ± 1.3 c | 64.9 ± 1.5 b | 23.6 ± 0.5 b |
| Co-P | 100.6 ± 1.2 b | 112.3 ± 2.4 b | 32.6 ± 1.5 c | 63.8 ± 1.5 b | 22.8 ± 0.5 b |
| Ca_Co-T | 83.2 ± 0.8 c | 95.3 ± 2.1 d | 35.9 ± 0.6 b | 45.7 ± 2.2 c | 17.3 ± 0.5 c,d |
| Ca_Co-P | 75.4 ± 0.6 d | 89.5 ± 1.2 e | 38.3 ± 1.2 a,b | 37.6 ± 2.5 d | 16.3 ± 0.7 d |
| Groups | AST (U/L) | ALT (U/L) | Albumin (g/dL) | Total Protein (g/dL) | Creatinine (mg/dL) | Urea (mg/dL) |
|---|---|---|---|---|---|---|
| NC | 47.67 ± 1.9 e | 37.4 ± 0.6 e | 3.8 ± 0.1 b,c | 7.28 ± 0.14 b | 0. 92 ± 0.1 d | 14. 4 ± 0.2 f |
| PC | 119.3 ± 1.6 a | 84.2 ± 1.0 a | 2.9 ± 0.03 d | 5.48 ± 0.14 f | 1.6 ± 0.1 a | 37.89 ± 0.2 a |
| Ca-T | 74.7 ± 1.0 d | 46.6 ± 0.7 c | 3.9 ± 0.1 b | 7.31 ± 0.1 b | 1.2 ± 0.1 b,c | 25.1 ± 0.4 c |
| Ca-P | 73.3 ± 0.6 d | 45.1 ± 1.6 c | 4.1 ± 0.1 a | 7.5 ± 0.13 a | 1.1 ± 0.07 c | 22.2 ± 0.2 d |
| Co-T | 99.3 ± 0.6 b | 51.7 ± 0.4 b | 3.6 ± 0.02 c | 6.94 ± 0.06 c | 1.3 ± 0.1 b | 27.57 ± 0.4 b |
| Co-P | 95.2 ± 0.5 b | 52.4 ± 0.4 b | 3. 5 ± 0.02 c | 6.5 ± 0.06 e | 1.3 ± 0.1 b | 26.23 ± 0.3 b |
| Ca_Co-T | 82.3 ± 0.63 c | 43.1 ± 0.7 c,d | 3.9 ± 0.1 b | 7.10 ± 0.1 d,e | 1.13 ± 0.1 c | 23 ± 0.2 d |
| Ca_Co-P | 74.45 ± 2.8 d | 41.3 ± 0.63 d | 4.2 ± 0.1 a | 7.76 ± 0.1 a | 1.01 ± 0.05 c,d | 19.5 ± 0.3 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, Y.M.; El-Zahar, K.M.; Mousa, H.M. Beneficial Effects of Fermented Camel and Cow’s Milk in Lipid Profile, Liver, and Renal Function in Hypercholesterolemic Rats. Fermentation 2022, 8, 171. https://doi.org/10.3390/fermentation8040171
Alharbi YM, El-Zahar KM, Mousa HM. Beneficial Effects of Fermented Camel and Cow’s Milk in Lipid Profile, Liver, and Renal Function in Hypercholesterolemic Rats. Fermentation. 2022; 8(4):171. https://doi.org/10.3390/fermentation8040171
Chicago/Turabian StyleAlharbi, Yousef Mesfer, Khaled Meghawry El-Zahar, and Hassan Mirghani Mousa. 2022. "Beneficial Effects of Fermented Camel and Cow’s Milk in Lipid Profile, Liver, and Renal Function in Hypercholesterolemic Rats" Fermentation 8, no. 4: 171. https://doi.org/10.3390/fermentation8040171
APA StyleAlharbi, Y. M., El-Zahar, K. M., & Mousa, H. M. (2022). Beneficial Effects of Fermented Camel and Cow’s Milk in Lipid Profile, Liver, and Renal Function in Hypercholesterolemic Rats. Fermentation, 8(4), 171. https://doi.org/10.3390/fermentation8040171