Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism
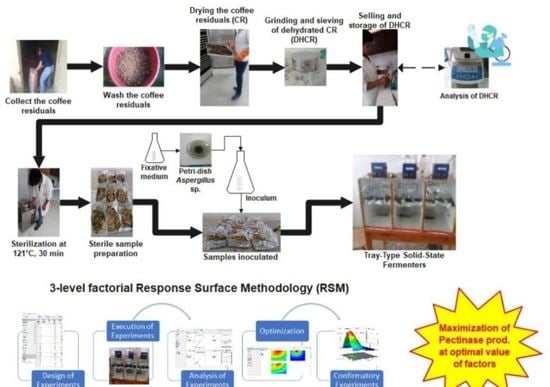
2.2. Raw Material and Inoculum Preparation
2.3. Spore Count and Pectinolytic Activity Analysis
2.4. Experimental Setup and Design
3. Results
3.1. Characterization of Dehydrated Coffee Pulp Residues
3.2. Three-Level Experiments Using the RSM to Maximize the Production of Pectinolytic Enzymes and the Concentration of Spores of the Aspergillus sp. on Dehydrated Coffee Residues by SSF
3.3. Validation of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Markets and Trade Coffee. Available online: https://www.fao.org/markets-and-trade/en/ (accessed on 6 March 2022).
- Padmapriya, R.; Tharian, J.A.; Thirunalasundari, T. Coffee waste management-An overview. Int. J. Curr. Sci. 2013, 33, 9–16. [Google Scholar]
- Mendoza Martinez, C.L.; Alves Rocha, E.P.; de Cassia Oliveira Carneiro, A.; Borges Gomes, F.J.; Ribas Batalha, L.A.; Vakkilainen, E.; Cardoso, M. Characterization of residual biomasses from the coffee production chain and assessment the potential for energy purposes. Biomass Bioenergy 2019, 120, 68–76. [Google Scholar] [CrossRef]
- Roussos, S.; Aquihuatl, M.A.; Trejo-Hernández, M.R.; Perraud, I.G.; Favela, E.; Ramakrishna, M.; Raimbault, M.; Viniegra-González, G. Biotechnological management of coffee pulp-isolation, screening, characterization, selection of caffeine-degrading fungi and natural microflora present in coffee pulp and husk. Appl. Microbiol. Biotechnol. 1995, 42, 756–762. [Google Scholar] [CrossRef]
- Kumar, S.S.; Swapna, T.S.; Sabu, A. Coffee Husk: A Potential Agro-Industrial Residue for Bioprocess. In Waste to Wealth; Reeta Rani, S., Rashmi Avinash, A., R. Praveen, K., Rajeev, K., Eds.; Springer: Singapore, 2018; pp. 97–109. ISBN 978-981-10-7430-1. [Google Scholar]
- Figueroa, G.A.; Homann, T.; Rawel, H.M. Coffee Production Wastes: Potentials and Perspectives. Austin Food Sci. 2016, 1, 10–14. [Google Scholar]
- Pérez-Sariñana, B.Y.; Saldaña-Trinidad, S. Chemistry and Biotransformation of Coffee By-Products to Biofuels. In The Question of Caffeine; Jolanta Natalia, L., Magdalena, L., Eds.; IntechOpen Ltd.: London, UK, 2017; pp. 143–161. [Google Scholar]
- Londoño-Hernandez, L.; Ruiz, H.A.; Cristina Ramírez, T.; Ascacio, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal detoxification of coffee pulp by solid-state fermentation. Biocatal. Agric. Biotechnol. 2020, 23, 101467. [Google Scholar] [CrossRef]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Santiago, A.M.; Conrado, L.S.; Mélo, B.C.A.; Sousa, C.A.B.; Oliveira, P.L.; Lima, F.C.S. Solid state fermentation: Fundamentals and application. In Transport Phenomena and Drying of Solids and Particulate Materials; Delgado, J.M.P.Q., Barbosa de Lima, A.G., Eds.; Springer: Cham, Switzerland, 2014; Volume 48, pp. 117–139. [Google Scholar]
- Lima, M.A.S.; De Oliveira, M.D.C.F.; Pimenta, A.T.Á.; Uchôa, P.K.S. Aspergillus niger: A hundred years of contribution to the natural products chemistry. J. Braz. Chem. Soc. 2019, 30, 2029–2059. [Google Scholar] [CrossRef]
- Ntana, F.; Mortensen, U.H.; Sarazin, C.; Figge, R. Aspergillus: A powerful protein production platform. Catalysts 2020, 10, 1064. [Google Scholar] [CrossRef]
- Uzuner, S.; Cekmecelioglu, D. Enzymes in the beverage industry. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Kuddus, M., Ed.; Academic Press: London, UK, 2018; pp. 29–43. ISBN 978-0-12-813280-7. [Google Scholar]
- Tapre, A.R.; Jain, R.K. Pectinases: Enzymes for fruit processing industry. Int. Food Res. J. 2014, 21, 447–453. [Google Scholar]
- Amin, F.; Bhatti, H.N.; Bilal, M. Recent advances in the production strategies of microbial pectinases—A review. Int. J. Biol. Macromol. 2019, 122, 1017–1026. [Google Scholar] [CrossRef]
- Cavalieri de Alencar Guimarães, N.; Glienke, N.N.; Silva Galeano, R.M.; Ruller, R.; Zanoelo, F.F.; Masui, D.C.; Giannesi, G.C. Polygalacturonase from Aspergillus japonicus (PGAj): Enzyme production using low-cost carbon source, biochemical properties and application in clarification of fruit juices. Biocatal. Agric. Biotechnol. 2022, 39, 102233. [Google Scholar] [CrossRef]
- Jacob, N. Pectinolytic enzymes. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; nee’ Nigam, P.S., Pandey, A., Eds.; Springer: Dordrecht, The Netjerlands, 2009; pp. 383–396. ISBN 978-1-4020-9941-0. [Google Scholar]
- Jayani, R.S.; Saxena, S.; Gupta, R. Microbial pectinolytic enzymes: A review. Process. Biochem. 2005, 40, 2931–2944. [Google Scholar] [CrossRef]
- Li, P.J.; Xia, J.-l.; Shan, Y.; Nie, Z.Y. Comparative study of multi-enzyme production from typical agro-industrial residues and ultrasound-assisted extraction of crude enzyme in fermentation with Aspergillus japonicus PJ01. Bioprocess Biosyst. Eng. 2015, 38, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Panwar, D.; Kaira, G.S. Bioprocesses for Enzyme Production Using Agro-Industrial Wastes: Technical Challenges and Commercialization Potential. In Agro-Industrial Wastes as Feedstock for Enzyme Production: Apply and Exploit the Emerging and Valuable Use Options of Waste Biomass; Singh, D.G., Kaur, S., Eds.; Academic Press: London, UK, 2016; pp. 61–93. ISBN 978-0-12-802392-1. [Google Scholar]
- Ojeda-Hernández, D.D.; Cosío-Cuadros, R.; Sandoval, G.; Rodríguez-González, J.A.; Mateos-Díaz, J.C. Solid-state fermentation as an economic production method of lipases. In Lipases and Phospholipases (Methods in Molecular Biology); Sandoval, G., Ed.; Humana Press: New York, NY, USA, 2018; Volume 1835, pp. 217–228. ISBN 978-1-4939-8671-2. [Google Scholar]
- Ortiz, G.E.; Ponce-Mora, M.C.; Noseda, D.G.; Cazabat, G.; Saravalli, C.; López, M.C.; Gil, G.P.; Blasco, M.; Albertó, E.O. Pectinase production by Aspergillus giganteus in solid-state fermentation: Optimization, scale-up, biochemical characterization and its application in olive-oil extraction. J. Ind. Microbiol. Biotechnol. 2017, 44, 197–211. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, A.; Mandalari, G.; Arena, N.; Nucita, F.; Tripodo, M.M.; Lo Curto, R.B. SCP and crude pectinase production by slurry-state fermentation of lemon pulps. Bioresour. Technol. 2002, 83, 89–94. [Google Scholar] [CrossRef]
- Heerd, D.; Yegin, S.; Tari, C.; Fernandez-Lahore, M. Pectinase enzyme-complex production by Aspergillus spp. in solid-state fermentation: A comparative study. Food Bioprod. Process. 2012, 90, 102–110. [Google Scholar] [CrossRef]
- Reichembach, L.H.; de Oliveira Petkowicz, C.L. Extraction and characterization of a pectin from coffee (Coffea arabica L.) pulp with gelling properties. Carbohydr. Polym. 2020, 245, 116473. [Google Scholar] [CrossRef] [PubMed]
- Manasa, V.; Padmanabhan, A.; Anu Appaiah, K.A. Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle. Waste Manag. 2021, 120, 762–771. [Google Scholar] [CrossRef]
- Sonawane, A.; Pathak, S.S.; Pradhan, R.C. Optimization of a Process for the Enzymatic Extraction of Nutrient Enriched Bael Fruit Juice Using Artificial Neural Network (ANN) and Response Surface Methodology (RSM). Int. J. Fruit Sci. 2020, 20, S1845–S1861. [Google Scholar] [CrossRef]
- Pais-Chanfrau, J.M.; Núñez-Pérez, J.; del Carmen Espin-Valladares, R.; Vinicio Lara-Fiallos, M.; Enrique Trujillo-Toledo, L. Uses of the Response Surface Methodology for the Optimization of Agro-Industrial Processes. In Response Surface Methodology in Engineering Science; Palanikumar Kayaroganam, Ed.; Intech: London, UK, 2021; pp. 1–21. ISBN 978-1-83968-918-5. [Google Scholar]
- Trejo-Hernández, M.R.; Oriol, E.; López-Canales, A.; Roussos, S.; Viniegra-González, G.; Raimbault, M. Producción de pectinasas de Aspergillus niger por fermentación sólida sobre soporte. Micol. Neotrop. Apl. 1991, 4, 49–62. [Google Scholar]
- Subhosh Chandra, M.G.; Rajasekhar Reddy, B. Exoglucanase production by Aspergillus niger grown on wheat bran: Exoglucanase by A. niger in SSF. Ann. Microbiol. 2013, 63, 871–877. [Google Scholar] [CrossRef]
- Singh, B.; Satyanarayana, T. Phytase production by a thermophilic mould Sporotrichum thermophile in solid state fermentation and its potential applications. Bioresour. Technol. 2008, 99, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Dhurba, G.; Ezhil, K. Manual Cell Counting with Neubauer Chamber. Available online: https://laboratoryinfo.com/manual-cell-counting-neubauer-chamber/ (accessed on 6 March 2022).
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Prajapati, J.; Dudhagara, P.; Patel, K. Production of thermal and acid-stable pectinase from Bacillus subtilis strain BK-3: Optimization, characterization, and application for fruit juice clarification. Biocatal. Agric. Biotechnol. 2021, 35, 102063. [Google Scholar] [CrossRef]
- Rodríguez Frómeta, R.A.; Sánchez, J.L.; Ros García, J.M. Evaluation of coffee pulp as substrate for polygalacturonase production in solid state fermentation. Emirates J. Food Agric. 2020, 32, 117–124. [Google Scholar] [CrossRef]
- Klingel, T.; Kremer, J.I.; Gottstein, V.; De Rezende, T.R.; Schwarz, S.; Lachenmeier, D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods 2020, 9, 665. [Google Scholar] [CrossRef]
- Oliveira, G.; Passos, C.P.; Ferreira, P.; Coimbra, M.A.; Gonçalves, I. Coffee by-products and their suitability for developing active food packaging materials. Foods 2021, 10, 683. [Google Scholar] [CrossRef]
- Do, P.M.N.; Hoang, Q.T.T.; Le, A.T.H. Optimization of conditions of extraction process from coffee cherry pulp (cascara) and application to drinking water products with antioxidant activity. Vietnam J. Sci. Technol. 2019, 57, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Cardona Alzate, C.A. The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing By-Products: Sustainable Applications; Galanakis, C.M., Ed.; Academic Press: London, UK, 2017; pp. 63–92. ISBN 978-0-12-811290-8. [Google Scholar]
- Zengin, G.; Sinan, K.I.; Mahomoodally, M.F.; Angeloni, S.; Mustafa, A.M.; Vittori, S.; Maggi, F.; Caprioli, G. Chemical composition, antioxidant and enzyme inhibitory properties of different extracts obtained from spent coffee ground and coffee silverskin. Foods 2020, 9, 713. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–246. [Google Scholar] [CrossRef]
- Antier, P.; Minjares, A.; Roussos, S.; Raimbault, M.; Viniegra-Gonzalez, G. Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enzyme Microb. Technol. 1993, 15, 254–260. [Google Scholar] [CrossRef]
- Patil, S.R.; Dayanand, A. Optimization of process for the production of fungal pectinases from deseeded sunflower head in submerged and solid-state conditions. Bioresour. Technol. 2006, 97, 2340–2344. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.K.; Nanda, P.K.; Sahoo, S. Enhanced production of pectinase by Aspergillus terreus NCFT 4269.10 using banana peels as substrate. 3 Biotech 2016, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoodi, M.; Najafpour, G.D.; Mohammadi, M. Bioconversion of agroindustrial wastes to pectinases enzyme via solid state fermentation in trays and rotating drum bioreactors. Biocatal. Agric. Biotechnol. 2019, 21, 101280. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.E.; Rodríguez-León, J.A.; de Carvalho, J.C.; Sturm, W.; Soccol, C.R. The behavior of kinetic parameters in production of pectinase and xylanase by solid-state fermentation. Bioresour. Technol. 2011, 102, 10657–10662. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Value 1 | Standard 2 | |
|---|---|---|
| Reducing sugars, wt.% | 4.0 ± 0.2 | AOAC Method 985.29 (NTE INEN 1707) |
| pH | 4.39 ± 0.14 | AOAC method 22.061 (NTE INEN 0381) |
| Ash | 9.69 ± 0.23 | AOAC method 925.51 (NTE INEN 0774) |
| Moisture, % | 8.83 ± 1.34 | ISO 6540 (NTE INEN 1513) |
| Dry matter, % | 91.17 ± 1.34 | ISO 6540 (NTE INEN 1513) |
| Run | Coded Factors | Actual Factors | Actual | Model | ||||
|---|---|---|---|---|---|---|---|---|
| A: Temp. | B: RH | Temp., °C | RH, % | S, #sp./gws | EA, IU/gws | S, #sp./gws | EA, IU/gws | |
| 1 | 0 | −1 | 35 | 40 | 5.00 × 103 | 12.30 | 3.83 × 103 | 12.41 |
| 2 | 0 | −1 | 35 | 40 | 3.50 × 103 | 10.79 | 3.83 × 103 | 12.41 |
| 3 | 0 | −1 | 35 | 40 | 3.25 × 103 | 14.14 | 3.83 × 103 | 12.41 |
| 4 | −1 | +1 | 25 | 80 | 2.40 × 105 | 15.86 | 2.41 × 105 | 16.87 |
| 5 | −1 | +1 | 25 | 80 | 2.39 × 105 | 18.13 | 2.41 × 105 | 16.87 |
| 6 | −1 | +1 | 25 | 80 | 2.44 × 105 | 16.94 | 2.41 × 105 | 16.87 |
| 7 | +1 | −1 | 45 | 40 | 1.25 × 103 | 12.30 | 1.17 × 103 | 12.08 |
| 8 | +1 | −1 | 45 | 40 | 1.75 × 103 | 12.73 | 1.17 × 103 | 12.08 |
| 9 | +1 | −1 | 45 | 40 | 0.75 × 103 | 11.44 | 1.17 × 103 | 12.08 |
| 10 | −1 | 0 | 25 | 60 | 1.93 × 105 | 30.32 | 1.91 × 105 | 22.96 |
| 11 | −1 | 0 | 25 | 60 | 1.80 × 105 | 20.18 | 1.91 × 105 | 22.96 |
| 12 | −1 | 0 | 25 | 60 | 1.99 × 105 | 19.64 | 1.91 × 105 | 22.96 |
| 13 | +1 | 0 | 45 | 60 | 1.45 × 104 | 15.75 | 1.91 × 104 | 14.67 |
| 14 | +1 | 0 | 45 | 60 | 2.15 × 104 | 15.86 | 1.91 × 104 | 14.67 |
| 15 | +1 | 0 | 45 | 60 | 2.28 × 104 | 12.41 | 1.91 × 104 | 14.67 |
| 16 | 0 | +1 | 35 | 80 | 3.48 × 106 | 30.21 | 3.17 × 106 | 29.31 |
| 17 | 0 | +1 | 35 | 80 | 2.88 × 106 | 28.38 | 3.17 × 106 | 29.31 |
| 18 | 0 | +1 | 35 | 80 | 3.18 × 106 | 28.16 | 3.17 × 106 | 29.31 |
| 19 | −1 | −1 | 25 | 40 | 3.25 × 103 | 14.46 | 4.80 × 103 | 15.20 |
| 20 | −1 | −1 | 25 | 40 | 6.50 × 103 | 19.10 | 4.80 × 103 | 15.20 |
| 21 | −1 | −1 | 25 | 40 | 5.25 × 103 | 13.16 | 4.80 × 103 | 15.20 |
| 22 | 0 | 0 | 35 | 60 | 7.03 × 105 | 24.93 | 8.31 × 105 | 22.25 |
| 23 | 0 | 0 | 35 | 60 | 8.54 × 105 | 20.50 | 8.31 × 105 | 22.25 |
| 24 | 0 | 0 | 35 | 60 | 9.55 × 105 | 23.31 | 8.31 × 105 | 22.25 |
| 25 | +1 | +1 | 45 | 80 | 4.28 × 104 | 19.21 | 4.85 × 104 | 17.20 |
| 26 | +1 | +1 | 45 | 80 | 4.85 × 104 | 17.59 | 4.85 × 104 | 17.20 |
| 27 | +1 | +1 | 45 | 80 | 5.58 × 104 | 15.43 | 4.85 × 104 | 17.20 |
| ANOVA for Quadratic Model of Transformed Spore Count (#sp./gws) | |||||
|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Model | 169.19 | 8 | 21.15 | 401 | <0.0001 |
| A-Temperature | 7.89 | 1 | 7.89 | 149.57 | <0.0001 |
| B-RH | 67.65 | 1 | 67.65 | 1282.66 | <0.0001 |
| AB | 0.028 | 1 | 0.028 | 0.5311 | 0.4755 |
| A2 | 13.73 | 1 | 13.73 | 260.31 | <0.0001 |
| B2 | 8.14 | 1 | 8.14 | 154.35 | <0.0001 |
| A2B | 8.4 | 1 | 8.4 | 159.23 | <0.0001 |
| AB2 | 0.6273 | 1 | 0.6273 | 11.89 | 0.0029 |
| A2B2 | 0.6373 | 1 | 0.6373 | 12.08 | 0.0027 |
| Pure Error | 0.9493 | 18 | 0.0527 | ||
| Cor Total | 170.14 | 26 | |||
| Std. Dev. | 0.2296 | R2 | 0.9944 | ||
| Mean | 10.85 | Adjusted R2 | 0.9919 | ||
| C.V. % | 2.12 | Predicted R2 | 0.9874 | ||
| Adeq. Precision | 59.5632 | ||||
| ANOVA for Quadratic Model of Transformed EA (IU/gws) | |||||
| Source | Sum of Squares | df | Mean Square | F-value | p-value |
| Model | 0.0291 | 7 | 0.0042 | 16.74 | <0.0001 |
| A-Temperature | 0.0041 | 1 | 0.0041 | 16.54 | 0.0007 |
| B-RH | 0.0147 | 1 | 0.0147 | 59.36 | <0.0001 |
| AB | 0.0009 | 1 | 0.0009 | 3.45 | 0.0790 |
| A2 | 0.0031 | 1 | 0.0031 | 12.63 | 0.0021 |
| B2 | 0.0030 | 1 | 0.0030 | 12.05 | 0.0026 |
| A2B | 0.0048 | 1 | 0.0048 | 19.40 | 0.0003 |
| AB2 | 0.0014 | 1 | 0.0014 | 5.82 | 0.0261 |
| Residual | 0.0047 | 19 | 0.0002 | ||
| Lack of Fit | 0.0000 | 1 | 0.0000 | 0.1705 | 0.6845 |
| Pure Error | 0.0047 | 18 | 0.0003 | ||
| Cor Total | 0.0338 | 26 | |||
| Std. Dev. | 0.0158 | R2 | 0.8605 | ||
| Mean | 0.2421 | Adjusted R2 | 0.8091 | ||
| C.V. % | 6.51 | Predicted R2 | 0.7129 | ||
| Adeq. Precision | 11.9954 | ||||
| Pred. Mean | Pred. Median 1 | Std Dev. | 95% PI low | Data Mean 2 | 95% PI high | |
|---|---|---|---|---|---|---|
| S | 3.35 × 106 | 3.27 × 106 | 0.78 × 106 | 2.23 × 106 | 3.64 × 106 | 4.78 × 106 |
| EA | 29.84 | 29.20 | 5.06 | 22.53 | 29.85 | 39.35 |
| Fungi | Substrate | Culture Conditions | Pectinase (IU/gds) | Ref. |
|---|---|---|---|---|
| Aspergillus niger C28B25 (Irradiate mutant) | Coffee pulp (sieving w/mesh 30) | Packed-bed glass cylinder SSF at 25 °C, 20 g w/moisture 60%, aeration rate: 60 mL min−1 saturated, for 72 h | 228.0 U/g 1 | [42] |
| Aspergillus niger DMF 45 | Deseeded sunflower head | SSF at 34 °C and pH 5.0, inoculum 107 sp./g, 500 µm size, 65% moisture | 34.2 | [43] |
| Aspergillus terreus (NCFT 4269.10) | Banana peel (Musa paradisiaca L.) | SSF at 30 ± 1 °C for 96 h | 36.1 ± 6.2 (6500 ± 1116 U/g) 2 | [44] |
| Aspergillus giganteus (NRRL10) | A mix of wheat bran: orange peel: lemon peel (66:17:17) | Tray-type SSF at 28 °C, pH 4.8 for 60 h, aeration rate: 20 L min−1 kgds−1 | 197 (PGase) 101 (PMGase) | [22] |
| Aspergillus niger | orange pomace peel + 40 g gds−1 bagasse | Tray-type SSF at 30 °C, moisture 60%, for 96 h | 49 (exo-PGase) 14 (endo-PGase) | [45] |
| Aspergillus sp. | DH coffee residuals | Tray-type SSF at 35 °C and 79% RH, 2 mm particles size | 85.3 ± 14.5 (29.9 ± 5.1) 3 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Núñez Pérez, J.; Chávez Arias, B.S.; de la Vega Quintero, J.C.; Zárate Baca, S.; Pais-Chanfrau, J.M. Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation. Fermentation 2022, 8, 170. https://doi.org/10.3390/fermentation8040170
Núñez Pérez J, Chávez Arias BS, de la Vega Quintero JC, Zárate Baca S, Pais-Chanfrau JM. Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation. Fermentation. 2022; 8(4):170. https://doi.org/10.3390/fermentation8040170
Chicago/Turabian StyleNúñez Pérez, Jimmy, Brayan Santiago Chávez Arias, Juan Carlos de la Vega Quintero, Santiago Zárate Baca, and José Manuel Pais-Chanfrau. 2022. "Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation" Fermentation 8, no. 4: 170. https://doi.org/10.3390/fermentation8040170
APA StyleNúñez Pérez, J., Chávez Arias, B. S., de la Vega Quintero, J. C., Zárate Baca, S., & Pais-Chanfrau, J. M. (2022). Multi-Objective Statistical Optimization of Pectinolytic Enzymes Production by an Aspergillus sp. on Dehydrated Coffee Residues in Solid-State Fermentation. Fermentation, 8(4), 170. https://doi.org/10.3390/fermentation8040170