Crabtree Effect on Rhodosporidium toruloides Using Wood Hydrolysate as a Culture Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate, Microorganism, and Inoculum Preparation
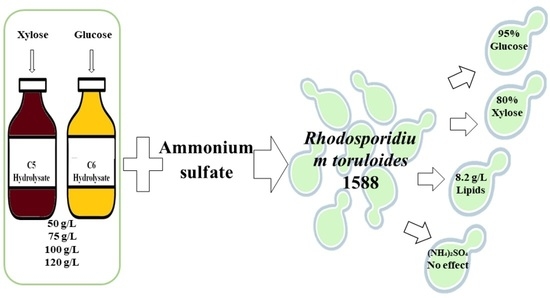
2.2. Sugar Concentration and Ammonium Sulfate Addition Effects
2.3. Cell Harvest and Lipid Extraction
2.4. Analytical Methods
2.4.1. Microbial Growth
2.4.2. Sugar Utilization
2.4.3. Lipid Content and Fatty Acid Determination
2.4.4. Total Nitrogen Content
2.5. Data Analysis
3. Results
3.1. Substrate Inhibition Analysis
3.2. Biomass Production Analysis
3.3. Nitrogen Effect
3.4. Lipid Accumulation and Fatty Acid Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bardi, L. Production of Bio-Oils from Microbial Biomasses. In Mycoremediation and Environmental Sustainability; Prasad, R., Ed.; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 61–89. ISBN 978-3-319-77385-8. [Google Scholar]
- Chaturvedi, S.; Bhattacharya, A.; Khare, S.K. Trends in Oil Production from Oleaginous Yeast Using Biomass: Biotechnological Potential and Constraints. Appl. Biochem. Microbiol. 2018, 54, 361–369. [Google Scholar] [CrossRef]
- Raud, M.; Kikas, T.; Sippula, O.; Shurpali, N.J. Potentials and Challenges in Lignocellulosic Biofuel Production Technology. Renew. Sustain. Energy Rev. 2019, 111, 44–56. [Google Scholar] [CrossRef]
- Zhu, P.; Abdelaziz, O.Y.; Hulteberg, C.P.; Riisager, A. New Synthetic Approaches to Biofuels from Lignocellulosic Biomass. Curr. Opin. Green Sustain. Chem. 2020, 21, 16–21. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A Review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Adrio, J.L. Oleaginous Yeasts: Promising Platforms for the Production of Oleochemicals and Biofuels: Microbial Production of Oleochemicals and Biofuels. Biotechnol. Bioeng. 2017, 114, 1915–1920. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Hegde, K.; Brar, S.K.; Kermanshahipour, A.; Avalos-Ramírez, A. Challenges in Lipid Production from Lignocellulosic Biomass Using Rhodosporidium Sp.; A Look at the Role of Lignocellulosic Inhibitors. Biofuels Bioprod. Bioref. 2019, 13, 740–759. [Google Scholar] [CrossRef]
- Park, Y.-K.; Nicaud, J.-M.; Ledesma-Amaro, R. The Engineering Potential of Rhodosporidium toruloides as a Workhorse for Biotechnological Applications. Trends Biotechnol. 2018, 36, 304–317. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative Lipid Production by Oleaginous Yeasts in Hydrolyzates of Lignocellulosic Biomass and Process Strategy for High Titers: High Titer Lipid Production by Yeasts in Lignocellulosic Hydrolyzates. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef] [Green Version]
- Tkáčová, J.; Klempová, T.; Čertík, M. Kinetic Study of Growth, Lipid and Carotenoid Formation in β-Carotene Producing Rhodotorula Glutinis. Chem. Pap. 2018, 72, 1193–1203. [Google Scholar] [CrossRef]
- Nair, A.S.; Sivakumar, N. Enhanced Production of Biodiesel by Rhodosporidium toruloides Using Waste Office Paper Hydrolysate as Feedstock: Optimization and Characterization. Fuel 2022, 327, 125174. [Google Scholar] [CrossRef]
- Tuhanioglu, A.; Hamamci, H.; Alpas, H.; Cekmecelioglu, D. Valorization of Apple Pomace Via Single Cell Oil Production Using Oleaginous Yeast Rhodosporidium toruloides. Waste Biomass Valor. 2022, 8, 1–15. [Google Scholar] [CrossRef]
- Vorapreeda, T.; Thammarongtham, C.; Cheevadhanarak, S.; Laoteng, K. Alternative Routes of Acetyl-CoA Synthesis Identified by Comparative Genomic Analysis: Involvement in the Lipid Production of Oleaginous Yeast and Fungi. Microbiology 2012, 158, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Coradetti, S.T.; Pinel, D.; Geiselman, G.M.; Ito, M.; Mondo, S.J.; Reilly, M.C.; Cheng, Y.-F.; Bauer, S.; Grigoriev, I.V.; Gladden, J.M.; et al. Functional Genomics of Lipid Metabolism in the Oleaginous Yeast Rhodosporidium toruloides. eLife 2018, 7, e32110. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; De Lucia, M.; Leonardi, A.; Rossi, M. Single Cell Oils of the Cold-Adapted Oleaginous Yeast Rhodotorula Glacialis DBVPG 4785. Microb. Cell Fact. 2010, 9, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dourou, M.; Aggeli, D.; Papanikolaou, S.; Aggelis, G. Critical Steps in Carbon Metabolism Affecting Lipid Accumulation and Their Regulation in Oleaginous Microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 2509–2523. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Hegde, K.; Ferreira, P.; Brar, S.K.; Kermanshahipour, A.; Soccol, C.R.; Avalos-Ramírez, A. Lipid Production in Rhodosporidium toruloides Using C-6 and C-5 Wood Hydrolysate: A Comparative Study. Biomass Bioenergy 2019, 130, 105355. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Saini, R.; Hegde, K.; Brar, S.K.; Lefebvre, A.; Avalos-Ramírez, A. Inhibitor Degradation by Rhodosporidium toruloides NRRL 1588 Using Undetoxified Wood Hydrolysate as a Culture Media. Biomass Bioenergy 2022, 160, 106419. [Google Scholar] [CrossRef]
- Osorio-González, C.S.; Saini, R.; Hegde, K.; Brar, S.K.; Avalos Ramirez, A. Furfural Degradation and Its Effect on Rhodosporidium toruloides-1588 during Microbial Growth and Lipid Accumulation. Bioresour. Technol. 2022, 359, 127496. [Google Scholar] [CrossRef]
- Saini, R.; Osorio-Gonzalez, C.S.; Hegde, K.; Brar, S.K.; Vezina, P. Effect of Creating a Fed-Batch like Condition Using Carbon to Nitrogen Ratios on Lipid Accumulation in Rhodosporidium toruloides-1588. Bioresour. Technol. 2021, 337, 125354. [Google Scholar] [CrossRef]
- Saini, R.; Osorio-Gonzalez, C.S.; Hegde, K.; Kaur Brar, S.; Vezina, P. A Co-Fermentation Strategy with Wood Hydrolysate and Crude Glycerol to Enhance the Lipid Accumulation in Rhodosporidium toruloides-1588. Bioresour. Technol. 2022, 364, 127821. [Google Scholar] [CrossRef]
- Saini, R.; Hegde, K.; Osorio-Gonzalez, C.S.; Brar, S.K.; Vezina, P. Evaluating the Potential of Rhodosporidium toruloides-1588 for High Lipid Production Using Undetoxified Wood Hydrolysate as a Carbon Source. Energies 2020, 13, 5960. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.-J. C/N Ratio and Carbon Source-Dependent Lipid Production Profiling in Rhodotorula Toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [Green Version]
- Lopes, H.J.S.; Bonturi, N.; Miranda, E.A. Induction of Resistance Mechanisms in Rhodotorula Toruloides for Growth in Sugarcane Hydrolysate with High Inhibitor Content. Appl. Microbiol. Biotechnol. 2021, 105, 9261–9272. [Google Scholar] [CrossRef]
- Tiukova, I.A.; Brandenburg, J.; Blomqvist, J.; Sampels, S.; Mikkelsen, N.; Skaugen, M.; Arntzen, M.Ø.; Nielsen, J.; Sandgren, M.; Kerkhoven, E.J. Proteome Analysis of Xylose Metabolism in Rhodotorula Toruloides during Lipid Production. Biotechnol. Biofuels 2019, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Tiukova, I.A.; Prigent, S.; Nielsen, J.; Sandgren, M.; Kerkhoven, E.J. Genome-scale Model of Rhodotorula Toruloides Metabolism. Biotechnol. Bioeng. 2019, 116, 3396–3408. [Google Scholar] [CrossRef]
- Ling, J.; Nip, S.; Shim, H. Enhancement of Lipid Productivity of Rhodosporidium toruloides in Distillery Wastewater by Increasing Cell Density. Bioresour. Technol. 2013, 146, 301–309. [Google Scholar] [CrossRef]
- Jagtap, S.S.; Rao, C.V. Production of D-Arabitol from d-Xylose by the Oleaginous Yeast Rhodosporidium toruloides IFO0880. Appl. Microbiol. Biotechnol. 2018, 102, 143–151. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Bai, F. High-Density Cultivation of Oleaginous Yeast Rhodosporidium toruloides Y4 in Fed-Batch Culture. Enzym. Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced Lipid Production by Rhodosporidium toruloides Using Different Fed-Batch Feeding Strategies with Lignocellulosic Hydrolysate as the Sole Carbon Source. Biotechnol. Biofuels 2016, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Maza, D.D.; Viñarta, S.C.; Su, Y.; Guillamón, J.M.; Aybar, M.J. Growth and Lipid Production of Rhodotorula Glutinis R4, in Comparison to Other Oleaginous Yeasts. J. Biotechnol. 2020, 310, 21–31. [Google Scholar] [CrossRef]




| Substrate | Initial Sugar (g) | S (g/L) | B (g/L) | YB/S (g/g) | µmax (h−1) | XSmax (h−1) | YL/S (g/g) |
|---|---|---|---|---|---|---|---|
| C6 hydrolysate + (NH4)2SO4 | 50 | 49.90 ± 0.01 | 17.00 ± 0.42 | 0.34 | 0.47 | 0.50 | 0.16 |
| C6 hydrolysate | 49.90 ± 0.01 | 14.50 ± 0.45 | 0.29 | 0.56 | 0.61 | 0.14 | |
| C5 hydrolysate + (NH4)2SO4 | 25.43 ± 2.82 | 11.00 ± 0.32 | 0.43 | 0.61 | 0.60 | 0.12 | |
| C5 hydrolysate | 23.46 ± 7.31 | 13.50 ± 1.13 | 0.58 | 0.17 | 0.88 | 0.06 | |
| C6 hydrolysate + (NH4)2SO4 | 75 | 74.82 ± 0.07 | 24.00 ± 0.63 | 0.32 | 0.31 | 0.74 | 0.11 |
| C6 hydrolysate | 73.09 ± 0.13 | 16.50 ± 0.14 | 0.23 | 0.36 | 2.49 | 0.11 | |
| C5 hydrolysate + (NH4)2SO4 | 45.00 ± 4.24 | 9.50 ± 0.21 | 0.21 | 0.25 | 0.56 | 0.06 | |
| C5 hydrolysate | 43.00 ± 3.25 | 15.00 ± 0.91 | 0.35 | 0.12 | 1.34 | 0.03 | |
| C6 hydrolysate + (NH4)2SO4 | 100 | 86.60 ± 1.54 | 21.50 ± 0.14 | 0.25 | 0.22 | 1.11 | 0.05 |
| C6 hydrolysate | 81.71 ± 1.36 | 16.50 ± 0.21 | 0.20 | 0.11 | 2.22 | 0.05 | |
| C5 hydrolysate + (NH4)2SO4 | 73.96 ± 1.44 | 9.50 ± 0.98 | 0.13 | 0.53 | 2.36 | 0.02 | |
| C5 hydrolysate | 72.00 ± 1.24 | 17.00 ± 0.63 | 0.24 | 0.22 | 2.57 | 0.02 | |
| C6 hydrolysate + (NH4)2SO4 | 120 | 97.00 ± 1.69 | 5.00 ± 0.14 | 0.05 | 0.28 | 2.92 | 0.05 |
| C6 hydrolysate | 96.00 ± 2.34 | 3.50 ± 0.77 | 0.04 | 0.08 | 3.19 | 0.02 | |
| C5 hydrolysate + (NH4)2SO4 | 95.00 ± 1.07 | 10.00 ± 1.48 | 0.11 | 0.22 | 2.64 | 0.03 | |
| C5 hydrolysate | 96.00 ± 1.21 | 13.50 ± 1.54 | 0.14 | 0.69 | 4.00 | 0.01 |
| Relative Fatty Acid Content (%) | C6 Wood Hydrolysate + Nitrogen | C6 Wood Hydrolysate | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Glucose Content (g/L) | Initial Xylose Content (g/L) | |||||||
| 50 | 75 | 100 | 120 | 50 | 75 | 100 | 120 | |
| Palmitic | 23.39 ± 1.16 | 23.37 ± 1.16 | 20.15 ± 1.00 | 21.20 ± 1.06 | 11.45 ± 0.57 | 23.06 ± 1.15 | 22.69 ± 1.13 | 19.99 ± 0.99 |
| Stearic | 14.92 ± 0.74 | 15.73 ± 0.78 | 13.09 ± 0.65 | 18.83 ± 0.94 | 17.91 ± 0.89 | 15.53 ± 0.77 | 11.97 ± 0.59 | 17.69 ± 0.88 |
| Oleic | 39.05 ± 1.95 | 44.60 ± 2.23 | 47.48 ± 2.37 | 34.74 ± 1.73 | 37.7 ± 1.88 | 35.33 ± 1.76 | 41.86 ± 2.09 | 35.76 ± 1.78 |
| Linoleic | 11.30 ± 0.56 | 6.28 ± 0.31 | 7.415 ± 0.37 | 8.31 ± 0.41 | 15.38 ± 0.76 | 12.19 ± 0.60 | 12.33 ± 0.61 | 8.96 ± 0.44 |
| Linolenate | 3.26 ± 0.16 | 1.92 ± 0.09 | 3.09 ± 0.15 | 5.10 ± 0.25 | 8.40 ± 0.42 | 5.91 ± 0.29 | 3.38 ± 0.16 | 5.41 ± 0.27 |
| Lignoceric | 2.19 ± 0.10 | 2.36 ± 0.11 | 2.38 ± 0.11 | 3.88 ± 0.19 | 1.54 ± 0.07 | 1.47 ± 0.07 | 1.79 ± 0.08 | 3.74 ± 0.18 |
| C5 wood hydrolysate + nitrogen | C5 wood hydrolysate | |||||||
| Palmitic | 17.50 ± 0.87 | 17.62 ± 0.88 | 17.4 ± 0.87 | 16.49 ± 0.82 | 16.72 ± 0.83 | 16.89 ± 0.84 | 17.47 ± 0.87 | 17.81 ± 0.89 |
| Stearic | 16.73 ± 0.83 | 18.25 ± 0.91 | 17.64 ± 0.88 | 18.17 ± 0.90 | 17.01 ± 0.85 | 17.08 ± 0.85 | 17.81 ± 0.89 | 19.40 ± 0.97 |
| Oleic | 41.49 ± 2.07 | 41.28 ± 2.06 | 40.35 ± 2.01 | 40.18 ± 2.00 | 41.60 ± 2.08 | 41.63 ± 2.08 | 38.8 ± 1.94 | 38.67 ± 1.93 |
| Linoleic | 8.45 ± 0.42 | 7.345 ± 0.36 | 8.04 ± 0.40 | 7.87 ± 0.39 | 8.28 ± 0.41 | 8.16 ± 0.40 | 7.77 ± 0.38 | 6.96 ± 0.34 |
| Linolenate | 5.05 ± 0.25 | 4.565 ± 0.22 | 5.15 ± 0.25 | 4.73 ± 0.23 | 4.94 ± 0.24 | 4.99 ± 0.24 | 4.73 ± 0.23 | 3.90 ± 0.19 |
| Lignoceric | 3.47 ± 0.17 | 3.345 ± 0.16 | 3.51 ± 0.17 | 3.45 ± 0.17 | 3.77 ± 0.18 | 3.56 ± 0.17 | 3.23 ± 0.16 | 3.70 ± 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osorio-González, C.S.; Saini, R.; Hegde, K.; Brar, S.K.; Lefebvre, A.; Avalos Ramírez, A. Crabtree Effect on Rhodosporidium toruloides Using Wood Hydrolysate as a Culture Media. Fermentation 2023, 9, 11. https://doi.org/10.3390/fermentation9010011
Osorio-González CS, Saini R, Hegde K, Brar SK, Lefebvre A, Avalos Ramírez A. Crabtree Effect on Rhodosporidium toruloides Using Wood Hydrolysate as a Culture Media. Fermentation. 2023; 9(1):11. https://doi.org/10.3390/fermentation9010011
Chicago/Turabian StyleOsorio-González, Carlos S., Rahul Saini, Krishnamoorthy Hegde, Satinder Kaur Brar, Alain Lefebvre, and Antonio Avalos Ramírez. 2023. "Crabtree Effect on Rhodosporidium toruloides Using Wood Hydrolysate as a Culture Media" Fermentation 9, no. 1: 11. https://doi.org/10.3390/fermentation9010011
APA StyleOsorio-González, C. S., Saini, R., Hegde, K., Brar, S. K., Lefebvre, A., & Avalos Ramírez, A. (2023). Crabtree Effect on Rhodosporidium toruloides Using Wood Hydrolysate as a Culture Media. Fermentation, 9(1), 11. https://doi.org/10.3390/fermentation9010011