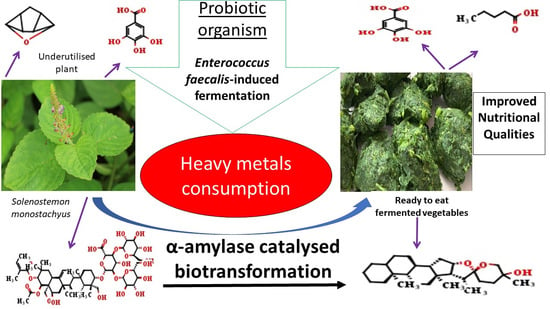
Enterococcus faecalis-Induced Biochemical Transformation during Fermentation of Underutilized Solenostemon monostachyus Leaves
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection of Plant Leaves
2.3. Procedure for Preparation of Organism for the Experimentation
Collection and Sub-Culturing of Lactic Acid Bacteria
2.4. Procedure for the Fermentation of Plant Leaves
2.4.1. Preparation of the Plant Leaves for Fermentation
2.4.2. Sterilization of Materials for Use during Fermentation
2.5. Post-Fermentation Analyses
2.5.1. Quantitative Proximate Composition and Total Phytochemical Analysis
2.5.2. Assay for Total Antioxidant Capacity (TAC) and Ferric-Reducing Antioxidant Power (FRAP)
2.5.3. Diphenyl-1-Picrylhydrazyl Radical Scavenging Activity
2.5.4. Assay for Micronutrients in the Sample Leaves
Procedure for Preparing the Samples for Mineral Analysis
Assay for Vitamin Contents of the Sample Leaves with HPLC
Water Soluble Vitamin C Analysis
2.5.5. Assay for Phytochemical Constituents of the Sample Leaves
Preparation of Sample for HPLC Analysis
Procedure for the HPLC Analysis of the Saponins Fractions
Procedure for the HPLC Analysis of the Phenolic Fractions
Procedure for the HPLC Analysis of the Flavonoids Fractions
Procedure for the Phytochemical Analysis Using GC-MS
2.5.6. Procedure for Enzyme Analysis
α-Amylase Activity Assay
Lactate Dehydrogenase Activity Assay
2.6. Method of Statistical Analysis
3. Results
3.1. Effect of Fermentation on the Acidity of the Leaves of S. monostachyus
3.2. Effect of Fermentation on the Nutritional Qualities of the Leaves of S. monostachyus
3.3. Effect of Fermentation on the Antioxidant Qualities of the Leaves of S. monostachyus
3.4. Effect of Fermentation on the Phytochemical Constituents of the Leaves of S. monostachyus
3.5. Metabolism of Phytochemicals during the Fermentation of the Leaves of S. monostachyus
3.6. Effect of Fermentation on the Biochemical Status of the Leaves of S. monostachyus
4. Discussion
4.1. Effect of Fermentation on the Nutritional Qualities of the Leaves of S. monostachyus
4.2. Total Antioxidants Qualities
4.3. Effect of Fermentation on the Phytochemical Constituents of the Leaves of S. monostachyus
4.4. Effect of Fermentation on the Enzyme Activities of the Leaves of S. monostachyus
4.4.1. Involvement of LDH Activity in the Fermentation
4.4.2. Involvement of α-Amylase Activity in the Fermentation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Solomons, N.W. Fermentation, fermented foods and lactose intolerance. Eur. J. Clin. Nutr. 2002, 56, S50–S55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taveira, I.C.; Nogueira, K.M.V.; Oliveira, D.L.G.d.; Silva, R.d.N. Fermentation: Humanity’s oldest biotechnological tool. Front. Young Minds 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Codjoe, S.N.; Okutu, D.; Abu, M. Urban household characteristics and dietary diversity: An analysis of food security in Accra, Ghana. Food Nutr. Bull. 2016, 37, 202–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matemilola, S.; Elegbede, I. The challenges of food security in Nigeria. OALib 2017, 4, 1–22. [Google Scholar] [CrossRef]
- Scarborough, M.J.; Hamilton, J.J.; Erb, E.A.; Donohue, T.J.; Noguera, D.R. Diagnosing and predicting mixed culture fermentations with unicellular and guild-based metabolic models. mSystems 2020, 5, e00755-20. [Google Scholar] [CrossRef]
- Feng, Y.; Albiol Tapia, M.; Okada, K.; Castaneda Lazo, N.B.; Chapman-Novakofski, K.; Phillips, C.; Lee, S.Y. Consumer acceptance comparison between seasoned and unseasoned vegetables. J. Food Sci. 2018, 83, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Fritts, J.R.; Fort, C.; Quinn Corr, A.; Liang, Q.; Alla, L.; Cravener, T.; Hayes, J.E.; Rolls, B.J.; D’Adamo, C.; Keller, K.L. Herbs and spices increase liking and preference for vegetables among rural high school students. Food Qual. Preference 2018, 68, 125–134. [Google Scholar] [CrossRef]
- Thomas, A.; Boobyer, C.; Borgonha, Z.; van den Heuvel, E.; Appleton, K.M. Adding flavours: Use of and attitudes towards sauces and seasonings in a sample of community-dwelling UK older adults. Foods 2021, 10, 2828. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Hamdi, M. Microbiological, biochemical, and functional aspects of fermented vegetable and fruit beverages. J. Chem. 2020, 2020, 5790432. [Google Scholar] [CrossRef]
- Ehigiator, B.E.; Adesida, A.S. Chicoric acid, a phytochemical compound of Solenostemon monostachyus: Possible drug candidate for the relief of erectile dysfunction. Int. J. Eng. Appl. Sci. Technol. 2020, 4, 509–518. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Osikoya, I.O.; Fajimi, O.D.; Usoro, P.I.; Ogunleye, D.O.; Bisi-Adeniyi, T.; Adeyemi, O.A.; Adekeye, B.T. Solenostemon monostachyus, Ipomoea involucrata and Carica papaya seed oil versus Glutathione, or Vernonia amygdalina: Methanolic extracts of novel plants for the management of sickle cell anemia disease. BMC Complement. Altern. Med. 2012, 12, 262. [Google Scholar] [CrossRef] [Green Version]
- Brenes-Prendas, S.; Agüero-Alvarado, R.; Sánchez-Vindas, P.; Poveda-Álvarez, L. Primer reporte de la arvense Solenostemon monostachyus (P. Beauv.) Briq. (Fam. Lamiaceae) en agroecosistemas de Costa Rica. Agron. Mesoam. 2013, 24, 427–431. [Google Scholar] [CrossRef] [Green Version]
- Webb, P.; Stordalen, G.A.; Singh, S.; Wijesinha-Bettoni, R.; Shetty, P.; Lartey, A. Hunger and malnutrition in the 21st century. BMJ 2018, 361, k2238. [Google Scholar] [CrossRef] [Green Version]
- Takahata, M.; Frémont, M.; Desreumaux, P.; Rousseaux, C.; Dubuquoy, C.; Shimomiya, Y.; Nakamura, Y.; Miyake, Y. Evaluation of therapeutic properties of fermented vegetables extract (OM-X®) in the model of colitis induced by Citrobacter rodentium in mice. J. Funct. Foods 2014, 10, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Park, K.Y.; Jeong, J.K.; Lee, Y.E.; Daily, J.W., 3rd. Health benefits of kimchi (korean fermented vegetables) as a probiotic food. J. Med. Food 2014, 17, 6–20. [Google Scholar] [CrossRef]
- Olusa, A.S.; Oyemitan, I.A. Acute toxicity test and behavioural activity of aqueous and ethanol dried leaf extracts of Solenostemon monostachyus in mice. Niger. J. Pharm. Res. 2021, 17, 1–14. [Google Scholar] [CrossRef]
- Odutayo, O.E.; Adegboye, B.E.; Omonigbehin, E.A.; Olawole, T.D.; Ogunlana, O.O.; Afolabi, I.S. Structural transformation and creativity induced by biological agents during fermentation of edible nuts from Terminalia catappa. Molecules 2021, 26, 5874. [Google Scholar] [CrossRef]
- Devi, S.M.; Archer, A.C.; Halami, P.M. Screening, characterization and In vitro evaluation of probiotic properties among lactic acid bacteria through comparative analysis. Probiotics Antimicrob. Proteins 2015, 7, 181–192. [Google Scholar] [CrossRef]
- Stoll, D.A.; Wafula, E.N.; Mathara, J.M.; Trierweiler, B.; Kulling, S.E.; Huch, M. Fermentation of African nightshade leaves with lactic acid bacterial starter cultures. Int. J. Food Microbiol. 2021, 342, 109056. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Oloyede, O.B. Biochemical response of sweet potato to bemul-wax coating combined with calcium chloride treatment during ambient storage. Afr. J. Biotechnol. 2011, 10, 2724–2732. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis; Association of Official Analytical Chemists, Inc.: Boulevard Arlington, VA, USA, 1990. [Google Scholar]
- Kalaydzhiev, H.; Ivanova, P.; Uzunova, G.; Manolov, I.; Chalova, V. Biuret and Bradford methods suitability for protein quantification in rapeseed meal. Contemp. Agric. 2018, 67, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, M.H.; Jaafar, H.Z.E. Involvement of carbohydrate, protein and phenylanine ammonia lyase in up-regulation of secondary metabolites in Labisia pumila under various CO2 and N2 level. Molecules 2011, 16, 4172–4190. [Google Scholar] [CrossRef] [Green Version]
- Olawole, T.D.; Olalere, A.T.; Adeyemi, O.A.; Okwumabua, O.; Afolabi, I.S. Tannin and antioxidant status of fermented and dried Sorghum bicolor. Rasāyan J. Chem. 2019, 12, 523–530. [Google Scholar] [CrossRef]
- Sharma, D.C.; Shukla, R.; Ali, J.; Sharma, S.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antioxidant assay, antibacterial activity and determination of cell viability (J774 and THP1 alpha cell lines) of P. sylvestris leaf crude and methanol purified fractions. EXCLI J. 2016, 15, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sakat, S.; Juvekar, A. Comparative study of Erythrina indica Lam. (Febaceae) leaves extracts for antioxidant activity. J. Young Pharm. 2010, 2, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Boyer, K.W. Metals and other Elements at Trace Levels in Foods; Association of Official Analytical Chemists, Inc.: Boulevard Arlington, VA, USA, 1984; pp. 444–476. [Google Scholar]
- Afolabi, I.S.; Nwachukwu, I.C.; Ezeoke, C.S.; Woke, R.C.; Adegbite, O.A.; Olawole, T.D.; Martins, O.C. Production of a new plant-based milk from Adenanthera pavonina seed and evaluation of its nutritional and health benefits. Front. Nutr. 2018, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Ho, J.C.K.; Yin Sze, L. Isolation, identification and characterization of enzyme-producing lactic acid bacteria from traditional fermented foods. Biosci. Horiz. Int. J. Stud. Res. 2018, 11, 1–11. [Google Scholar] [CrossRef]
- FAO. Human Vitamin and Mineral Requirements; FAO of the United Nations, Ed.; Food and Agricultural Organisation of the United Nations: Rome, Italy, 2001; p. 303. [Google Scholar]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, nutrition, metabolism, bioavailability, and health benefits in lettuce-A comprehensive review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef]
- Yakubu, C.M.; Sharma, R.; Sharma, S.; Singh, B. Influence of alkaline fermentation time on in vitro nutrient digestibility, bio- & techno-functionality, secondary protein structure and macromolecular morphology of locust bean (Parkia biglobosa) flour. LWT 2022, 161, 113295. [Google Scholar] [CrossRef]
- Anal, A. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [Green Version]
- Bautista-Exposito, S.; Penas, E.; Silvan, J.M.; Frias, J.; Martinez-Villaluenga, C. pH-controlled fermentation in mild alkaline conditions enhances bioactive compounds and functional features of lentil to ameliorate metabolic disturbances. Food Chem. 2018, 248, 262–271. [Google Scholar] [CrossRef]
- Ouoba, L.I.I.; Vouidibio Mbozo, A.B.; Anyogu, A.; Obioha, P.I.; Lingani-Sawadogo, H.; Sutherland, J.P.; Jespersen, L.; Ghoddusi, H.B. Environmental heterogeneity of Staphylococcus species from alkaline fermented foods and associated toxins and antimicrobial resistance genetic elements. Int. J. Food Microbiol. 2019, 311, 108356. [Google Scholar] [CrossRef] [Green Version]
- De Vuyst, L.; Vaningelgem, F.; Ghijsels, V.; Tsakalidou, E.; Leroy, F. New insights into the citrate metabolism of Enterococcus faecium FAIR-E 198 and its possible impact on the production of fermented dairy products. Int. Dairy J. 2011, 21, 580–585. [Google Scholar] [CrossRef]
- Martino, G.P.; Perez, C.E.; Magni, C.; Blancato, V.S. Implications of the expression of Enterococcus faecalis citrate fermentation genes during infection. PLoS ONE 2018, 13, e0205787. [Google Scholar] [CrossRef]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wolber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from food, clinical specimens, and oral sites: Prevalence of virulence factors in association with biofilm formation. Front. Microbiol. 2015, 6, 1534. [Google Scholar] [CrossRef] [Green Version]
- Wajda, Ł.; Ostrowski, A.; Błasiak, E.; Godowska, P. Enterococcus faecium isolates present in human breast milk might be carriers of multi-antibiotic resistance genes. Bacteria 2022, 1, 66–87. [Google Scholar] [CrossRef]
- Baccouri, O.; Boukerb, A.M.; Farhat, L.B.; Zebre, A.; Zimmermann, K.; Domann, E.; Cambronel, M.; Barreau, M.; Maillot, O.; Rince, I.; et al. Probiotic potential and safety evaluation of Enterococcus faecalis OB14 and OB15, isolated from traditional Tunisian testouri cheese and rigouta, using physiological and genomic analysis. Front. Microbiol. 2019, 10, 881. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, H.; Shigwedha, N.; Zhang, S.; Liu, F.; Zhang, L. Probiotic effects and metabolic products of Enterococcus faecalis LD33 with respiration capacity. Foods 2022, 11, 606. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of iron in plant growth and metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Fatima, G.; Raza, A.M.; Hadi, N.; Nigam, N.; Mahdi, A.A. Cadmium in human diseases: It’s more than just a mere metal. Indian J. Clin. Biochem. 2019, 34, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, M.; Saxena, J.; Nema, R.; Singh, D.; Gupta, A. Phytochemistry of medicinal plants. J. Pharmacogn. Phytochem. 2013, 1, 168–182. [Google Scholar]
- Hussein, W.E.; Abdelhamid, A.G.; Rocha-Mendoza, D.; Garcia-Cano, I.; Yousef, A.E. Assessment of safety and probiotic traits of Enterococcus durans OSY-EGY, isolated from Egyptian artisanal cheese, using comparative genomics and phenotypic analyses. Front. Microbiol. 2020, 11, 608314. [Google Scholar] [CrossRef] [PubMed]
- Dapkevicius, M.L.E.; Sgardioli, B.; Camara, S.P.A.; Poeta, P.; Malcata, F.X. Current trends of Enterococci in dairy products: A comprehensive review of their multiple roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Afolabi, I.S.; Uchendu, J.O.; Mustapha, O.S. Antioxidant and phytochemical qualities of Solenostermon monostachyus (SoleMon). Rasāyan J. Chem. 2021, 14, 2154–2160. [Google Scholar] [CrossRef]
- Ayoola, G.A.; Coker, H.A.; Adesegun, S.A.; Adepoju-Bello, A.A.; Obaweya, K.; Ezennia, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of saponins on lipid metabolism: A review of potential health benefits in the treatment of obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef] [Green Version]
- Podolak, I.; Galanty, A.; Sobolewska, D. Saponins as cytotoxic agents: A review. Phytochem. Rev. Proc. Phytochem. Soc. Eur. 2010, 9, 425–474. [Google Scholar] [CrossRef] [Green Version]
- Afolabi, I.S.; Jolaoluwa, A.F.; Awogbindin, V.O.; Amosun, P.T. Phytonutrients and bioactive compounds in the leaves of Solenostemon monostachyus. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Roberto Thewes, F.; Both, V.; Brackmann, A.; Rodrigo Thewes, F.; Junior Soldateli, F.; Roberto Pasquetti Berghetti, M.; Ludwig, V.; Mallmann Wendt, L.; Ribas Schiefelbein, H. Dynamic and static drying temperatures for ‘Barton’ pecans: Impacts on the volatile compounds profile and kernel color. LWT 2022, 161, 113393. [Google Scholar] [CrossRef]
- Liang, M.; Li, W.; Qi, Q.; Zeng, P.; Zhou, Y.; Zheng, Y.; Wu, M.; Ni, H. Catalyst for the degradation of 1,1-dimethylhydrazine and its by-product N-nitrosodimethylamine in propellant wastewater. RSC Adv. 2016, 6, 5677–5687. [Google Scholar] [CrossRef]
- Mu, X.; Wang, X.; Zhang, Y.; Liu, B.; Yang, J. Major products and their formation and transformation mechanism through degrading UDMH wastewater via DBD low temperature plasma. Environ. Technol. 2021, 42, 2709–2720. [Google Scholar] [CrossRef]
- Dani, V.; Goel, A.; Vaiphei, K.; Dhawan, D.K. Chemopreventive potential of zinc in experimentally induced colon carcinogenesis. Toxicol. Lett. 2007, 171, 10–18. [Google Scholar] [CrossRef]
- Jain, K.; Dhawan, D.K. Regulation of biokinetics of (65)Zn by curcumin and zinc in experimentally induced colon carcinogenesis in rats. Cancer Biother. Radiopharm. 2014, 29, 310–316. [Google Scholar] [CrossRef]
- Chari, K.Y.; Polu, P.R.; Shenoy, R.R. An appraisal of pumpkin seed extract in 1, 2-dimethylhydrazine induced colon cancer in wistar rats. J. Toxicol. 2018, 2018, 6086490. [Google Scholar] [CrossRef] [Green Version]
- Tudosie, M.; Pauna, A.; Stefani, C.; Staicu, I. Diet and food chemicals increasing the risk of colorectal cancer—Literature review. J. Mind Med. Sci. 2022, 9, 118–124. [Google Scholar] [CrossRef]
- Katsuki, T.; Hirata, K.; Ishikawa, H.; Matsuura, N.; Sumi, S.; Itoh, H. Aged garlic extract has chemopreventative effects on 1,2-dimethylhydrazine-induced colon tumors in rats. J. Nutr. 2006, 136, 847S–851S. [Google Scholar] [CrossRef] [Green Version]
- Torabi, F.; Dadkhah, A.; Fatemi, F.; Dini, S.; Taghizadeh, M.; Malayeri, M.R.M. Prevention and therapy of 1,2-dimethyl hydrazine induced colon carcinogenesis by Ferula assafoetida hydroalcoholic extract / 1,2-dimetilhidrazin ile indüklenmiş kalın bağırsak kanserlerinin Ferula assa-foetida ekstraktı kullanılarak önlenmesi ve tedavisi. Turk. J. Biochem. 2015, 40, 390–400. [Google Scholar] [CrossRef]
- Chikezie, P.C.; Ojiako, O.A. Cyanide and aflatoxin loads of processed cassava (Manihot esculenta) tubers (garri) in Njaba, Imo State, Nigeria. Toxicol. Int. 2013, 20, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Ihedioha, J.I. The clinicopathologic significance of enriching grated cassava mash with red palm oil in the production of gari. Plant Foods Hum. Nutr. 2002, 57, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Uvere, P.O. Reactivity of red palm oil and cyanide ion: Implications for the cyanogen content of palm oil-treated gari. Plant Foods Hum. Nutr. 1999, 53, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hucherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef] [PubMed]
- Odutayo, O.E.; Omonigbehin, E.A.; Olawole, T.D.; Ogunlana, O.O.; Afolabi, I.S. Fermentation enhanced biotransformation of compounds in the kernel of Chrysophyllum albidum. Molecules 2020, 25, 6021. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, R.; McCubbin, T.; Navone, L.; Stowers, C.; Nielsen, L.; Marcellin, E. Microbial propionic acid production. Fermentation 2017, 3, 21. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, B.S.; Sang, B.I. An efficient new process for the selective production of odd-chain carboxylic acids by simple carbon elongation using Megasphaera hexanoica. Sci. Rep. 2019, 9, 11999. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, G.; Manyar, H. Production pathways of acetic acid and its versatile applications in the food industry. In Biotechnological Applications of Biomass; Basso, T.P., Basso, T.O., Basso, L.C., Eds.; IntechOpen Limited: London, UK, 2021; p. 11. [Google Scholar]
- Song, J.; Wang, J.; Wang, X.; Zhao, H.; Hu, T.; Feng, Z.; Lei, Z.; Li, W.; Zheng, Y.; Wang, M. Improving the acetic acid fermentation of Acetobacter pasteurianus by enhancing the energy metabolism. Front. Bioeng. Biotechnol. 2022, 10, 815614. [Google Scholar] [CrossRef]
- Zhang, J.; Barajas, J.F.; Burdu, M.; Wang, G.; Baidoo, E.E.; Keasling, J.D. Application of an acyl-coA ligase from Streptomyces aizunensis for lactam biosynthesis. ACS Synth. Biol. 2017, 6, 884–890. [Google Scholar] [CrossRef] [Green Version]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric acid suppresses liver cancer development by acting as a novel HDAC inhibitor. Mol. Ther. Oncolytics 2020, 19, 8–18. [Google Scholar] [CrossRef]
- Gio-Batta, M.; Spetz, K.; Barman, M.; Braback, L.; Norin, E.; Bjorksten, B.; Wold, A.E.; Sandin, A. Low concentration of fecal valeric acid at 1 year of age is linked with eczema and food allergy at 13 years of age: Findings from a Swedish birth cohort. Int. Arch. Allergy Immunol. 2022, 183, 398–408. [Google Scholar] [CrossRef]
- Duan, G.; Liu, Y.; Lv, H.; Wu, F.; Wang, R. Optimization of “Zaoheibao” wine fermentation process and analysis of aroma substances. Biotechnol. Biotechnol. Equip. 2020, 34, 1056–1064. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, M.; Wu, S.; Zou, X.; Chen, X.; Ge, L.; Zhang, Q. Effects of gallic acid on fermentation parameters, protein fraction, and bacterial community of whole plant soybean silage. Front. Microbiol. 2021, 12, 662966. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Chen, C.; Xiang, J.Y.; Hu, W.; Xie, Y.B.; Wang, T.J.; Cui, J.W.; Xu, Y.; Liu, Z.; Xiang, H.; Xie, Q. Identification of key micro-organisms involved in Douchi fermentation by statistical analysis and their use in an experimental fermentation. J. Appl. Microbiol. 2015, 119, 1324–1334. [Google Scholar] [CrossRef] [Green Version]
- Meng, N.; Shao, J.; Li, H.; Wang, Y.; Fu, X.; Liu, C.; Yu, Y.; Zhang, B. Electrosynthesis of formamide from methanol and ammonia under ambient conditions. Nat. Commun. 2022, 13, 5452. [Google Scholar] [CrossRef]
- Guo, C.; Zhou, W.; Lan, X.; Wang, Y.; Li, T.; Han, S.; Yu, Y.; Zhang, B. Electrochemical upgrading of formic acid to formamide via coupling nitrite co-reduction. J. Am. Chem. Soc. 2022, 144, 16006–16011. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Brindisi, M. Organic carbamates in drug design and medicinal chemistry. J. Med. Chem. 2015, 58, 2895–2940. [Google Scholar] [CrossRef] [Green Version]
- Passarella, S.; Schurr, A. L-lactate transport and metabolism in mitochondria of hep G2 cells-The Cori cycle revisited. Front. Oncol. 2018, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.G.; Tipton, K.F. Parameter reliability and understanding enzyme function. Molecules 2022, 27, 263. [Google Scholar] [CrossRef]
- Kim, M.J.; Whitesides, G.M. L-Lactate dehydrogenase: Substrate specificity and use as a catalyst in the synthesis of homochiral 2-hydroxy acids. J. Am. Chem. Soc. 2002, 110, 2959–2964. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Singh, B.P.; Kulshrestha, S. Probiotic fermentation of polyphenols: Potential sources of novel functional foods. Food Prod. Process. Nutr. 2022, 4, 21. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Asgeirsson, B.; Rao, B.J. A measure of the broad substrate specificity of enzymes based on ’duplicate’ catalytic residues. PLoS ONE 2012, 7, e49313. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Xu, Q.; Liu, Z.; Jain, N.; Tyagi, M.; Wei, D.Q.; Hong, L. Controlling the substrate specificity of an enzyme through structural flexibility by varying the salt-bridge density. Molecules 2021, 26, 5693. [Google Scholar] [CrossRef]
- Scopes, R.K. Enzyme activity and assays. Encycl. Life Sci. 2002, 1–6. [Google Scholar] [CrossRef]
- de Souza, P.M.; de Oliveira Magalhaes, P. Application of microbial alpha-amylase in industry—A review. Braz. J. Microbiol. 2010, 41, 850–861. [Google Scholar] [CrossRef]
- Unban, K.; Kanpiengjai, A.; Takata, G.; Uechi, K.; Lee, W.C.; Khanongnuch, C. Amylolytic enzymes acquired from L-lactic acid producing Enterococcus faecium K-1 and improvement of direct lactic acid production from cassava starch. Appl. Biochem. Biotechnol. 2017, 183, 155–170. [Google Scholar] [CrossRef]



| Parameters | Leaf Types | Duration of Fermentation (Days) | ||
|---|---|---|---|---|
| 0 | 3 | 5 | ||
| pH | WL | 4.310 ± 0.017 a | 7.500 ± 0.000 b | 8.000 ± 0.000 c |
| SM | 4.623 ± 0.040 a | 8.047 ± 0.081 b | 8.610 ±0.017 c | |
| Moisture (%) | WL | 11.62 ± 0.03 a | 20.77 ± 0.06 b | 23.49 ± 0.00 c |
| SM | 13.30 ± 0.11 a | 11.00 ± 0.36 b | 10.46 ± 0.04 c | |
| Protein (%) | WL | 12.20 ± 0.10 a | 21.40 ± 0.10 b | 12.13 ± 0.06 c |
| SM | 13.07 ± 0.06 a | 20.47 ± 0.45 b | 23.07 ± 0.06 c | |
| Fats (%) | WL | 14.34 ± 0.11 a | 19.10 ± 0.17 b | 29.90 ± 0.09 c |
| SM | 16.94 ± 0.10 a | 12.82 ± 0.28 b | 7.20 ± 0.08 c | |
| Carbohydrates (%) | WL | 17.54 ± 0.04 a | 18.36 ± 0.13 b | 16.98 ± 0.04 c |
| SM | 15.83 ± 0.07 a | 19.98 ± 0.01 c | 16.24 ± 0.01 c | |
| Crude fiber (%) | WL | 32.56 ± 0.01 a | 10.01 ± 0.01 b | 11.22 ± 0.01 c |
| SM | 25.10 ± 0.05 a | 14.43 ± 0.02 b | 20.01 ± 0.01 c | |
| Ash (%) | WL | 11.73 ± 0.01 a | 10.35 ± 0.01 b | 6.27 ± 0.01 c |
| SM | 15.76 ± 0.02 a | 21.30 ± 0.01 b | 23.02 ± 0.01 c | |
| Minerals | Leaf Types | Duration of Fermentation (Days) | |||
|---|---|---|---|---|---|
| 0 | 3 | 5 | Recommended Daily Intake † | ||
| Ca (mg/L) | WL | 0.507 ± 0.000 | NA | NA | 1000 mg |
| SM | 0.967 ± 0.002 | 0.969 ± 0.001 | NA | ||
| Se (mg/L) | WL | 0.226 ± 0.000 | NA | NA | 25–34 μg |
| SM | 0.293 ± 0.002 a | 0.289 ± 0.000 b | NA | ||
| Mg (mg/L) | WL | 0.692 ± 0.002 | NA | NA | 400 mg |
| SM | 1.003 ± 0.002 | 0.998 ± 0.002 | NA | ||
| K (mg/L) | WL | 1.932 ± 0.000 a | NA | NA | 3500 mg |
| SM | 2.530 ± 0.010 | 2.540 ± 0.000 | NA | ||
| Na (mg/L) | WL | 0.485 ± 0.000 | NA | NA | 2400 mg |
| SM | 0.720 ± 0.000 | 0.719 ± 0.000 | NA | ||
| Zn (mg/L) | WL | 0.801 ± 0.000 | NA | NA | 15 mg |
| SM | 0.916 ± 0.000 | 0.916 ± 0.0000 | NA | ||
| Cu (mg/L) | WL | 0.190 ± 0.000 a | 0.275 ± 0.161 | 0.000 ± 0.000 b | 2 mg |
| SM | 0.420 ± 0.015 a | 0.264 ± 0.000 b | 0.207 ± 0.0118 c | ||
| Fe (mg/L) | WL | 8.728 ± 0.066 a | 16.524 ± 0.112 b | 0.000 ± 0.000 c | 18 mg |
| SM | 15.379 ± 0.130 a | 11.576 ± 0.000 b | 10.351 ± 0.000 c | ||
| Cd (mg/L) | WL | 0.045 ± 0.000 a | 0.050 ± 0.000 | 0.000 ± 0.000 b | 3.6 μg/kg bw |
| SM | 0.068 ± 0.030 a | 0.027 ± 0.023 b | 0.046 ± 0.000 c | ||
| Mn (mg/L) | WL | 1.241 ± 0.000 a | 0.911 ± 0.010 | 0.000 ± 0.000 b | 2 mg |
| SM | 0.707 ± 0.000 a | 1.016 ± 0.010 | 0.509 ± 0.000 a | ||
| Pb (mg/L) | WL | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.0 μg/kg bw |
| SM | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | ||
| Vit. A (mg/mL) | WL | 0.132 ± 0.000 | NA | NA | 5000 (I.U.) |
| SM | 0.016 ± 0.001 | 0.010 ± 0.000 | NA | ||
| Vit. C (mg/mL) | WL | 0.610 ± 0.000 | NA | NA | 60 mg |
| SM | 0.820 ± 0.006 a | 1.059 ± 0.023 b | NA | ||
| Vit. E (mg/mL) | WL | 0.821 ± 0.000 | NA | NA | 30 (I.U.) |
| SM | 1.547 ± 0.064 a | 0.321 ± 0.009 b | NA | ||
| Days | Phenol (mgGAE/g) × 10−2 | Saponin (mgGAE/g) | Flavonoids (mgGAE/g) | TAC (µg/mL) | FRAP (µg/mL) × 10−5 | DPPH Inhibition (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WL | SM | WL | SM | WL | SM | WL | SM | WL | SM | WL | SM | |
| 0 | 2.008 ± 0.007 a | 1.858 ± 0.024 a | 1.61 ± 0.17 a | 1.90 ± 0.08 a | 0.05 ± 0.00 | 0.04 ± 0.00 | 26.38 ± 0.001 a | 25.76 ± 0.02 a | 24.10 ± 0.00 a | 6.71 ± 0.06 a | 75.92 ± 0.25 a | 83.53 ± 0.74 a |
| 3 | 2.085 ± 0.007 b | 1.951 ± 0.019 b | 2.47 ± 0.32 b | 2.67 ± 0.05 b | 0.05 ± 0.00 | 0.05 ± 0.00 | 25.89 ± 0.02 b | 26.01 ± 0.02 b | 8.49 ± 0.00 b | 7.05 ± 0.01 b | 70.52 ± 0.00 b | 72.48 ± 0.25 b |
| 5 | 2.202 ± 0.007 c | 1.983 ± 0.031 c | 2.39 ± 0.51 b | 2.77 ± 0.04 b | 0.06 ± 0.00 | 0.05 ± 0.00 | 26.51 ± 0.02 c | 27.10 ± 0.02 c | 6.72 ± 0.01 c | 8.42 ± 0.01 c | 80.02 ± 0.14 c | 71.75 ± 0.25 c |
| S/N | Identified Compounds (Tr (min)Peak nos.) | Concentration (mg/10 g Extract) | |
|---|---|---|---|
| Saponins | Control (Day 0) | 5-Days Fermented | |
| 1 | S-S1 (0.9903–0.9486) | 0.011 | 1.313 |
| 2 | Spirostanol (1.6828) | - | 20.734 |
| 3 | P-S1 (1.8489) | - | 33.577 |
| 4 | Furostanol (2.5238–2.53210) | 29.420 | 19.873 |
| 5 | Aescin (2.5739) | 69.510 | - |
| 6 | P-S2 (3.90711) | - | 23.572 |
| Phenolic | Control (Day 0) | 5-Days Fermented | |
| 7 | Gallic acid (1.2152–1.3732) | 88.111 | 98.949 |
| 8 | Luteolin (3.4733–3.3653) | 11.712 | 0.954 |
| 9 | Kaempferol (4.6654) | 0.066 | - |
| 10 | Chrysin (6.1325) | 0.071 | - |
| S/N | Peaks | Tr | Area (%) | Similarity Index (%) | Class of Compound | IUPAC Name | Common Name |
|---|---|---|---|---|---|---|---|
| Unfermented—Day 0 | |||||||
| 1 | 1 | 4.333 | 48.83 | 65 | Cycloalkanes | 4,7-Epoxytricyclo [4.1.0.0(3,5)]heptane | Not applicable |
| 2 | 2 | 9.416 | 51.17 | 77 | Nitrile | 2-(Cyanomethylamino)pentanedinitrile | Possible novel compound |
| Fermented—Day 3 | |||||||
| 1 | 1 | 4.383 | 24.68 | 73 | Hydroxylamines | 2-Hydroxy-N-methylpropanamide | Not applicable |
| 2 | 2 | 5.191 | 28.19 | 84 | Fatty acid | Ethanoic acid | Acetic acid |
| 3 | 3 | 9.108 | 47.13 | 75 | Fatty acid ester | Carbamic acid, phenyl ester | Phenyl carbamate |
| Fermented—Day 5 | |||||||
| 1 | 1 | 5.090 | 13.00 | 67 | Fatty acid | 1-Butanecarboxylic acid | Valeric acid |
| 2 | 2 | 5.679 | 87.00 | 89 | Hydrazines | 1,2-Dimethylhydrazine | Hydrazomethane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afolabi, I.S.; Ahuekwe, E.F.; Garuba, P.A.; Adigun, A.J.; Odutayo, O.E.; Adeyemi, A.O. Enterococcus faecalis-Induced Biochemical Transformation during Fermentation of Underutilized Solenostemon monostachyus Leaves. Fermentation 2023, 9, 33. https://doi.org/10.3390/fermentation9010033
Afolabi IS, Ahuekwe EF, Garuba PA, Adigun AJ, Odutayo OE, Adeyemi AO. Enterococcus faecalis-Induced Biochemical Transformation during Fermentation of Underutilized Solenostemon monostachyus Leaves. Fermentation. 2023; 9(1):33. https://doi.org/10.3390/fermentation9010033
Chicago/Turabian StyleAfolabi, Israel Sunmola, Eze Frank Ahuekwe, Precious Amaneshi Garuba, Aderinsola Jumai Adigun, Oluwatofunmi E. Odutayo, and Alaba Oladipupo Adeyemi. 2023. "Enterococcus faecalis-Induced Biochemical Transformation during Fermentation of Underutilized Solenostemon monostachyus Leaves" Fermentation 9, no. 1: 33. https://doi.org/10.3390/fermentation9010033
APA StyleAfolabi, I. S., Ahuekwe, E. F., Garuba, P. A., Adigun, A. J., Odutayo, O. E., & Adeyemi, A. O. (2023). Enterococcus faecalis-Induced Biochemical Transformation during Fermentation of Underutilized Solenostemon monostachyus Leaves. Fermentation, 9(1), 33. https://doi.org/10.3390/fermentation9010033