A Chemical-Free Pretreatment for Biosynthesis of Bioethanol and Lipids from Lignocellulosic Biomass: An Industrially Relevant 2G Biorefinery Approach
Abstract
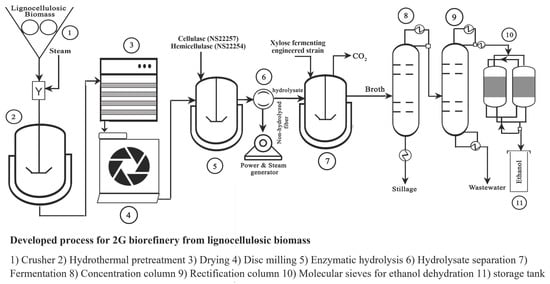
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Pretreatment
Compositional Analysis of Hydrothermal Pretreated Biomass
2.3. Enzymatic Hydrolysis
2.4. Fermentation
2.4.1. Microorganisms and Seed Culture Preparation
2.4.2. Fermentation of Enzymatic Hydrolysates for Bioethanol Production
2.4.3. Fermentation of Enzymatic Hydrolysates for Microbial Lipids Production
2.5. Analytical Methods
3. Result and Discussion
3.1. Effect of Hydrothermal Pretreatment Determined by Compositional Analysis
3.2. Enzymatic Hydrolysis
Effect of Citrate Buffer Strength on Enzymatic Hydrolysis
3.3. Ethanol Fermentation
3.3.1. Effect of Citrate Buffer Strength on Bioethanol Production
3.3.2. Microbial Lipid Production Cultures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keasling, J.; Garcia Martin, H.; Lee, T.S.; Mukhopadhyay, A.; Singer, S.W.; Sundstrom, E. Microbial Production of Advanced Biofuels. Nat. Rev. Microbiol. 2021, 19, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Field, J.L.; Richard, T.L.; Smithwick, E.A.H.; Cai, H.; Laser, M.S.; LeBauer, D.S.; Long, S.P.; Paustian, K.; Qin, Z.; Sheehan, J.J.; et al. Robust Paths to Net Greenhouse Gas Mitigation and Negative Emissions via Advanced Biofuels. Proc. Natl. Acad. Sci. USA 2020, 117, 21968–21977. [Google Scholar] [CrossRef] [PubMed]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol Production: Feedstock and Current Technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Kim, S.M.; Dien, B.S.; Singh, V. Promise of Combined Hydrothermal/Chemical and Mechanical Refining for Pretreatment of Woody and Herbaceous Biomass. Biotechnol. Biofuels 2016, 9, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.H.; Dien, B.S.; Lee, D.K.; Singh, V. Sugar Production from Bioenergy Sorghum by Using Pilot Scale Continuous Hydrothermal Pretreatment Combined with Disk Refining. Bioresour. Technol. 2019, 289, 121663. [Google Scholar] [CrossRef]
- Cheng, M.H.; Kadhum, H.J.; Murthy, G.S.; Dien, B.S.; Singh, V. High Solids Loading Biorefinery for the Production of Cellulosic Sugars from Bioenergy Sorghum. Bioresour. Technol. 2020, 318, 124051. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mogili, N.V.; Dutta, M.; Goswami, L.; Kushwaha, A.; Veeranki, V.D.; Goud, V.V. Role of Lignocellulosic Bioethanol in the Transportation Sector: Limitations and Advancements in Bioethanol Production from Lignocellulosic Biomass. In Waste-to-Energy Approaches Towards Zero Waste; Elsevier: Amsterdam, The Netherlands, 2022; pp. 57–85. ISBN 9780323853873. [Google Scholar]
- Wan, G.; Zhang, Q.; Li, M.; Jia, Z.; Guo, C.; Luo, B.; Wang, S.; Min, D. How Pseudo-Lignin Is Generated during Dilute Sulfuric Acid Pretreatment. J. Agric. Food Chem. 2019, 67, 10116–10125. [Google Scholar] [CrossRef]
- Qin, L.; Li, W.C.; Liu, L.; Zhu, J.Q.; Li, X.; Li, B.Z.; Yuan, Y.J. Inhibition of Lignin-Derived Phenolic Compounds to Cellulase. Biotechnol. Biofuels 2016, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Pisithkul, T.; Jacobson, T.B.; O’Brien, T.J.; Stevenson, D.M.; Amador-Noguez, D. Phenolic Amides Are Potent Inhibitors of de Novo Nucleotide Biosynthesis. Appl. Environ. Microbiol. 2015, 81, 5761–5772. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Veeranki, V.D.; Goud, V.V. Lignocellulosic Feedstocks for the Production of Bioethanol: Availability, Structure, and Composition. In Sustainable Bioenergy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–19. ISBN 9780128176542. [Google Scholar]
- Tarabanko, N.; Baryshnikov, S.V.; Kazachenko, A.S.; Miroshnikova, A.V.; Skripnikov, A.M.; Lavrenov, A.V.; Taran, O.P.; Kuznetsov, B.N. Hydrothermal Hydrolysis of Microcrystalline Cellulose from Birch Wood Catalyzed by Al2O3-B2O3 Mixed Oxides. Wood Sci. Technol. 2022, 56, 437–457. [Google Scholar] [CrossRef]
- Cheng, M.H.; Dien, B.S.; Jin, Y.S.; Thompson, S.; Shin, J.; Watson Slininger, P.J.; Qureshi, N.; Singh, V. Conversion of High-Solids Hydrothermally Pretreated Bioenergy Sorghum to Lipids and Ethanol Using Yeast Cultures. ACS Sustain. Chem. Eng. 2021, 9, 8515–8525. [Google Scholar] [CrossRef]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Moser, B.R.; Orjuela, A.; da Costa Sousa, L.; Balan, V. Microbial Lipid-Based Lignocellulosic Biorefinery: Feasibility and Challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.; Jin, Y.S. Production of Fuels and Chemicals from Xylose by Engineered Saccharomyces Cerevisiae: A Review and Perspective. Microb. Cell Fact. 2017, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.S.; Kong, I.I.; Lesmana, A.; Million, G.; Zhang, G.C.; Kim, S.R.; Jin, Y.S. Rapid and Marker-Free Refactoring of Xylose-Fermenting Yeast Strains with Cas9/CRISPR. Biotechnol. Bioeng. 2015, 112, 2406–2411. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Goud, V.V.; Veeranki, V.D. Liquefaction of Lignocellulosic Biomass through Biochemical Conversion Pathway: A Strategic Approach to Achieve an Industrial Titer of Bioethanol. Fuel 2021, 287, 119545. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Goud, V.V.; Veeranki, V.D. Curtailing Citrate Buffer Inhibition Effect on S. Cerevisiae to Enhance the Fermentability of Cellulosic Hydrolysate. J. Environ. Chem. Eng. 2021, 9, 105696. [Google Scholar] [CrossRef]
- Tao, L.; Schell, D.; Davis, R.; Tan, E.; Elander, R.; Bratis, A. NREL 2012 Achievement of Ethanol Cost Targets: Biochemical Ethanol Fermentation via Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2014.
- Arora, A.; Carrier, D.J. Understanding the Pine Dilute Acid Pretreatment System for Enhanced Enzymatic Hydrolysis. ACS Sustain. Chem. Eng. 2015, 3, 2423–2428. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C. Determination of Structural Carbohydrates and Lignin in Biomass Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2012.
- Deshavath, N.N.; Dasu, V.V.; Goud, V.V.; Rao, P.S. Development of Dilute Sulfuric Acid Pretreatment Method for the Enhancement of Xylose Fermentability. Biocatal. Agric. Biotechnol. 2017, 11, 224–230. [Google Scholar] [CrossRef]
- Dien, B.S.; Slininger, P.J.; Kurtzman, C.P.; Moser, B.R.; O’Bryan, P.J. Identification of Superior Lipid Producing Lipomyces and Myxozyma Yeasts. AIMS Environ. Sci. 2016, 3, 1–20. [Google Scholar] [CrossRef]
- Rodríguez-Zúñiga, U.F.; Cannella, D.; Giordano, R.D.C.; Giordano, R.D.L.C.; Jørgensen, H.; Felby, C. Lignocellulose Pretreatment Technologies Affect the Level of Enzymatic Cellulose Oxidation by LPMO. Green Chem. 2015, 17, 2896–2903. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Lv, Z.W.; Rao, J.; Tian, R.; Sun, S.N.; Peng, F. Effects of Hydrothermal Pretreatment on the Dissolution and Structural Evolution of Hemicelluloses and Lignin: A Review. Carbohydr. Polym. 2022, 281, 119050. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Silva, L.; López-González, D.; Villaseñor, J.; Sánchez, P.; Valverde, J.L. Thermogravimetric-Mass Spectrometric Analysis of Lignocellulosic and Marine Biomass Pyrolysis. Bioresour. Technol. 2012, 109, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mahanta, S.; Goud, V.V.; Dasu, V.V. Chemical Composition Analysis of Various Genetically Modified Sorghum Traits: Pretreatment Process Optimization and Bioethanol Production from Hemicellulosic Hydrolyzates without Detoxification. J. Environ. Chem. Eng. 2018, 6, 5625–5634. [Google Scholar] [CrossRef]
- Jing, X.; Zhang, X.; Bao, J. Inhibition Performance of Lignocellulose Degradation Products on Industrial Cellulase Enzymes during Cellulose Hydrolysis. Appl. Biochem. Biotechnol. 2009, 159, 696–707. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, D.; Huang, J.; Yu, Z.; Dai, G.; Bao, J. Simultaneous Saccharification and Ethanol Fermentation at High Corn Stover Solids Loading in a Helical Stirring Bioreactor. Biotechnol. Bioeng. 2010, 105, 718–728. [Google Scholar] [CrossRef]
- Jin, M.; Gunawan, C.; Uppugundla, N.; Balan, V.; Dale, B.E. A Novel Integrated Biological Process for Cellulosic Ethanol Production Featuring High Ethanol Productivity, Enzyme Recycling and Yeast Cells Reuse. Energy Environ. Sci. 2012, 5, 7168–7175. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.J.B.B.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2011.
- Casey, E.; Mosier, N.S.; Adamec, J.; Stockdale, Z.; Ho, N.; Sedlak, M. Effect of Salts on the Co-Fermentation of Glucose and Xylose by a Genetically Engineered Strain of Saccharomyces Cerevisiae. Biotechnol. Biofuels 2013, 6, 83. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.J.; Kim, M.D.; Ryu, Y.W.; Bisson, L.; Seo, J.H. Kinetic Studies on Glucose and Xylose Transport in Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2002, 60, 186–191. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive Response of Yeasts to Furfural and 5-Hydroxymethylfurfural and New Chemical Evidence for HMF Conversion to 2,5-Bis-Hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef]
- Lau, M.W.; Dale, B.E. Cellulosic Ethanol Production from AFEX-Treated Corn Stover Using Saccharomyces Cerevisiae 424A(LNH-ST). Proc. Natl. Acad. Sci. USA 2009, 106, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of Acetic Acid and PH on the Cofermentation of Glucose and Xylose to Ethanol by a Genetically Engineered Strain of Saccharomyces Cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Shen, G.; Chen, S.; Chen, X.; Zhang, C.; Liu, S.; Jin, M. Modified Simultaneous Saccharification and Co-Fermentation of DLC Pretreated Corn Stover for High-Titer Cellulosic Ethanol Production without Water Washing or Detoxifying Pretreated Biomass. Energy 2022, 247, 123488. [Google Scholar] [CrossRef]
- Shen, G.; Yuan, X.; Chen, S.; Liu, S.; Jin, M. High Titer Cellulosic Ethanol Production from Sugarcane Bagasse via DLCA Pretreatment and Process Development without Washing/Detoxifying Pretreated Biomass. Renew. Energy 2022, 186, 904–913. [Google Scholar] [CrossRef]
- Chen, X.; Kuhn, E.; Jennings, E.W.; Nelson, R.; Tao, L.; Zhang, M.; Tucker, M.P. DMR (Deacetylation and Mechanical Refining) Processing of Corn Stover Achieves High Monomeric Sugar Concentrations (230 g L−1) during Enzymatic Hydrolysis and High Ethanol Concentrations (>10% v/v) during Fermentation without Hydrolysate Purification or concentration. Energy Environ. Sci. 2016, 9, 1237–1245. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Q.; Li, H.; Qureshi, A.S.; Zhang, J.; Bao, X.; Bao, J. Dry Biorefining Maximizes the Potentials of Simultaneous Saccharification and Co-Fermentation for Cellulosic Ethanol Production. Biotechnol. Bioeng. 2018, 115, 60–69. [Google Scholar] [CrossRef]
- Cheng, M.H.; Sun, L.; Jin, Y.S.; Dien, B.; Singh, V. Production of Xylose Enriched Hydrolysate from Bioenergy Sorghum and Its Conversion to β-Carotene Using an Engineered Saccharomyces Cerevisiae. Bioresour. Technol. 2020, 308, 123275. [Google Scholar] [CrossRef]
- Ko, J.K.; Enkh-Amgalan, T.; Gong, G.; Um, Y.; Lee, S.M. Improved Bioconversion of Lignocellulosic Biomass by Saccharomyces Cerevisiae Engineered for Tolerance to Acetic Acid. GCB Bioenergy 2020, 12, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Slininger, P.J.; Thompson, S.R.; Weber, S.; Liu, Z.L.; Moon, J. Repression of Xylose-Specific Enzymes by Ethanol in Scheffersomyces (Pichia) Stipitis and Utility of Repitching Xylose-Grown Populations to Eliminate Diauxic Lag. Biotechnol. Bioeng. 2011, 108, 1801–1815. [Google Scholar] [CrossRef]
- Roca, C.; Olsson, L. Increasing Ethanol Productivity during Xylose Fermentation by Cell Recycling of Recombinant Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2003, 60, 560–563. [Google Scholar] [CrossRef]
- Olofsson, K.; Wiman, M.; Lidén, G. Controlled Feeding of Cellulases Improves Conversion of Xylose in Simultaneous Saccharification and Co-Fermentation for Bioethanol Production. J. Biotechnol. 2010, 145, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Juneja, A.; Singh, V. Fermentation Technology to Improve Productivity in Dry Grind Corn Process for Bioethanol Production. Fuel Process. Technol. 2018, 173, 66–74. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D. Exploitation of Genus Rhodosporidium for Microbial Lipid Production. World J. Microbiol. Biotechnol. 2017, 33, 54. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily Yeasts as Oleaginous Cell Factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative Lipid Production by Oleaginous Yeasts in Hydrolyzates of Lignocellulosic Biomass and Process Strategy for High Titers. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, Q.; O’Brien, M.; Nelson, R.; Chen, X.; Lowell, A.; Dowe, N. Enhanced Lipid Production by Rhodosporidium Toruloides Using Different Fed-Batch Feeding Strategies with Lignocellulosic Hydrolysate as the Sole Carbon Source. Biotechnol. Biofuels 2016, 9, 130. [Google Scholar] [CrossRef] [PubMed]








| Components (%w/w) * | Soxhlet Extracted | Without Soxhlet Extraction |
|---|---|---|
| Extractives | 35.5 ± 0.14 | |
| Water | ||
| Cellulose | 7.21 ± 0.06 † | |
| Xylan | 12.66 ± 0.03 ‡ | |
| Arabinan | 0.69 | |
| Acetic acid | 1.08 | |
| Ethanol | ||
| Cellulose | 5.56 ± 0.39 † | |
| Xylan | 4.31 ± 0.35 ‡ | |
| Acetic acid | 0.44 ± 0.036 | |
| Cellulose | 32.2 ± 0.4 † | 42.18 ± 0.1 |
| Hemicellulose | 8.21 | 25.07 |
| Xylan | 5.23 ± 0.17 ‡ | 15.9 ± 0.36 |
| Arabinan | 1.86 ± 0.012 | 6.75 ± 1.68 |
| Acetic acid | 1.12 ± 0.18 | 2.42 ± 0.17 |
| Lignin | 15.7 ± 1.12 | 28.8 ± 2.1 |
| Citrate Buffer (mM) | Cellobiose (g/L) | Glucose (g/L) | Xylose (g/L) | Arabinose (g/L) | Furfural (g/L) | 5-HMF (g/L) | Acetic Acid (g/L) | Formic Acid (g/L) | Levulinic Acid (g/L) |
|---|---|---|---|---|---|---|---|---|---|
| 50 | 6.55 ± 0.06 | 137.2 ± 0.17 | 78.4 ± 0.51 | 4.36 ± 0.05 | 0.06 ± 0.02 | 0.02 ± 0.007 | 11.2 ± 0.02 | 3.55 ± 0.01 | 2.90 ± 0.01 |
| 5 | 6.76 ± 0.05 | 137.8 ± 0.17 | 77.8 ± 0.21 | 4.39 ± 0.05 | 0.08 ± 0.01 | 0.01 ± 0.001 | 11.4 ± 0.01 | 3.5 ± 0.01 | 2.83 ± 0.04 |
| 0.5 | 7.72 ± 0.07 | 137.1 ± 0.15 | 77.4 ± 0.14 | 4.37 ± 0.03 | 0.08 ± 0.01 | 0.03 ± 0.001 | 11.4 ± 0.01 | 3.61 ± 0.05 | 2.83 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshavath, N.N.; Dien, B.S.; Slininger, P.J.; Jin, Y.-S.; Singh, V. A Chemical-Free Pretreatment for Biosynthesis of Bioethanol and Lipids from Lignocellulosic Biomass: An Industrially Relevant 2G Biorefinery Approach. Fermentation 2023, 9, 5. https://doi.org/10.3390/fermentation9010005
Deshavath NN, Dien BS, Slininger PJ, Jin Y-S, Singh V. A Chemical-Free Pretreatment for Biosynthesis of Bioethanol and Lipids from Lignocellulosic Biomass: An Industrially Relevant 2G Biorefinery Approach. Fermentation. 2023; 9(1):5. https://doi.org/10.3390/fermentation9010005
Chicago/Turabian StyleDeshavath, Narendra Naik, Bruce S. Dien, Patricia J. Slininger, Yong-Su Jin, and Vijay Singh. 2023. "A Chemical-Free Pretreatment for Biosynthesis of Bioethanol and Lipids from Lignocellulosic Biomass: An Industrially Relevant 2G Biorefinery Approach" Fermentation 9, no. 1: 5. https://doi.org/10.3390/fermentation9010005
APA StyleDeshavath, N. N., Dien, B. S., Slininger, P. J., Jin, Y. -S., & Singh, V. (2023). A Chemical-Free Pretreatment for Biosynthesis of Bioethanol and Lipids from Lignocellulosic Biomass: An Industrially Relevant 2G Biorefinery Approach. Fermentation, 9(1), 5. https://doi.org/10.3390/fermentation9010005